Men with recurrent prostate cancer following surgery or radiation therapy may now have a new chance to live longer. A massive global trial has found that a combination of two medications, enzalutamide and leuprolide, can lower the risk of death by more than 40 percent versus standard hormone therapy only.
Phase 3 EMBARK trial followed more than 1,000 men with biochemically recurrent prostate cancer and high risk for about eight years. The men did not have any visible evidence of spread on scan but had increasing levels of prostate-specific antigen (PSA), a protein that signals tumor activity, a sign that their disease most likely would recur in a serious fashion.
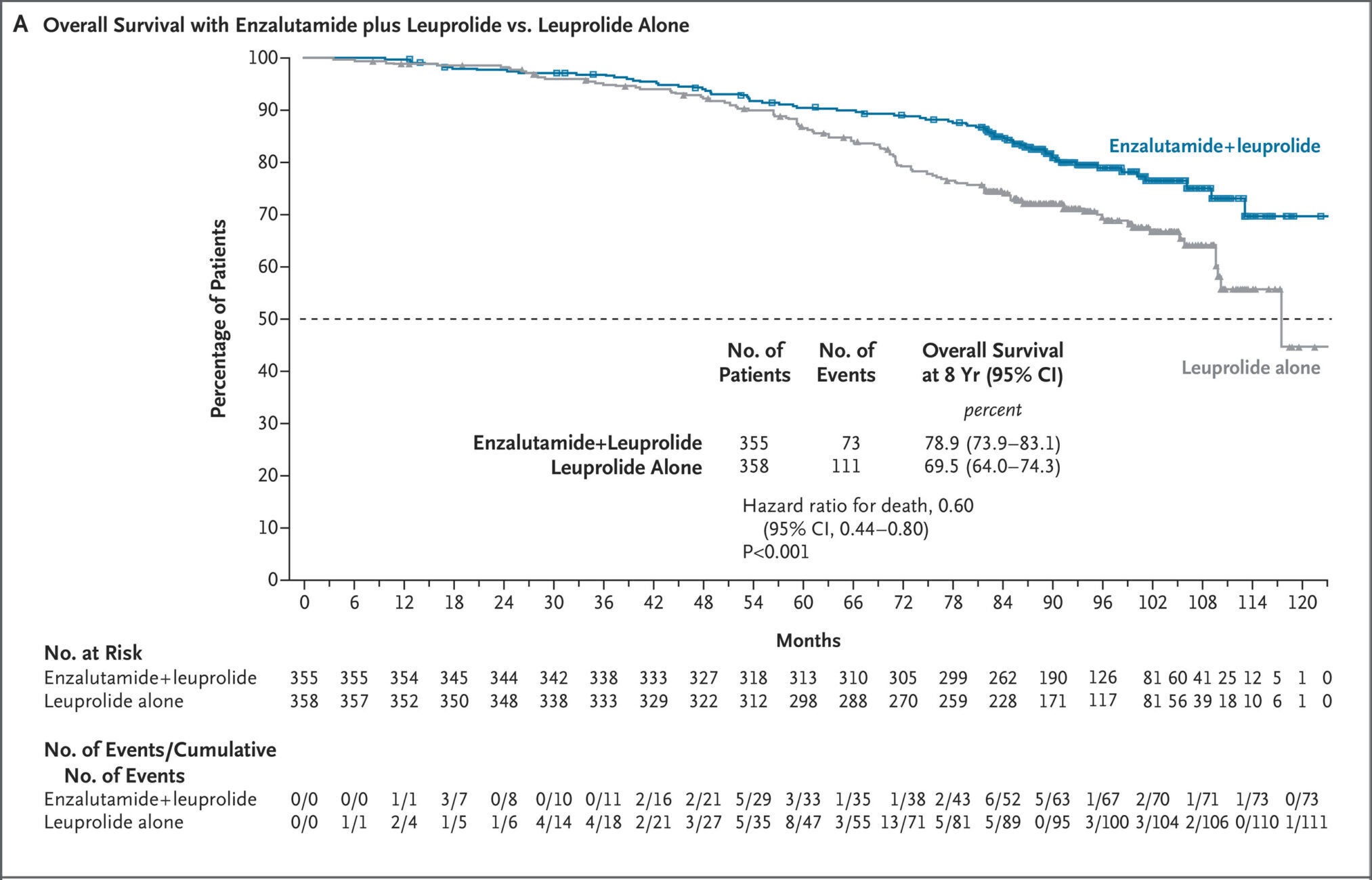
Scientists compared three treatment strategies to each other: leuprolide alone, enzalutamide alone, and both combined. Patients on the combination lived many years longer. Eight years after the study, 78.9 percent of the men taking the double therapy were alive, against 69.5 percent on leuprolide alone. That was a 40 percent lower risk of death.

“Happiness really is a game changer,” said Dr. Stephen Freedland, director of the Center for Integrated Research in Cancer and Lifestyle at Cedars-Sinai Cancer and one of the co-principal investigators on the study. “Hormone therapy has been our gold standard for 30 years, but it hasn’t improved survival—until now.”
The results, published in The New England Journal of Medicine and presented at the European Society for Medical Oncology Congress in Berlin, will likely set treatment for men whose prostate cancer recurs after initial treatment.
Between 2015 and 2018, 1,068 men from 244 sites in 17 countries were randomly assigned to one of the three groups. They all had rising PSA levels but no detectable metastases. Researchers employed “high-risk” as a definition to signify a doubling of PSA levels within nine months or less.
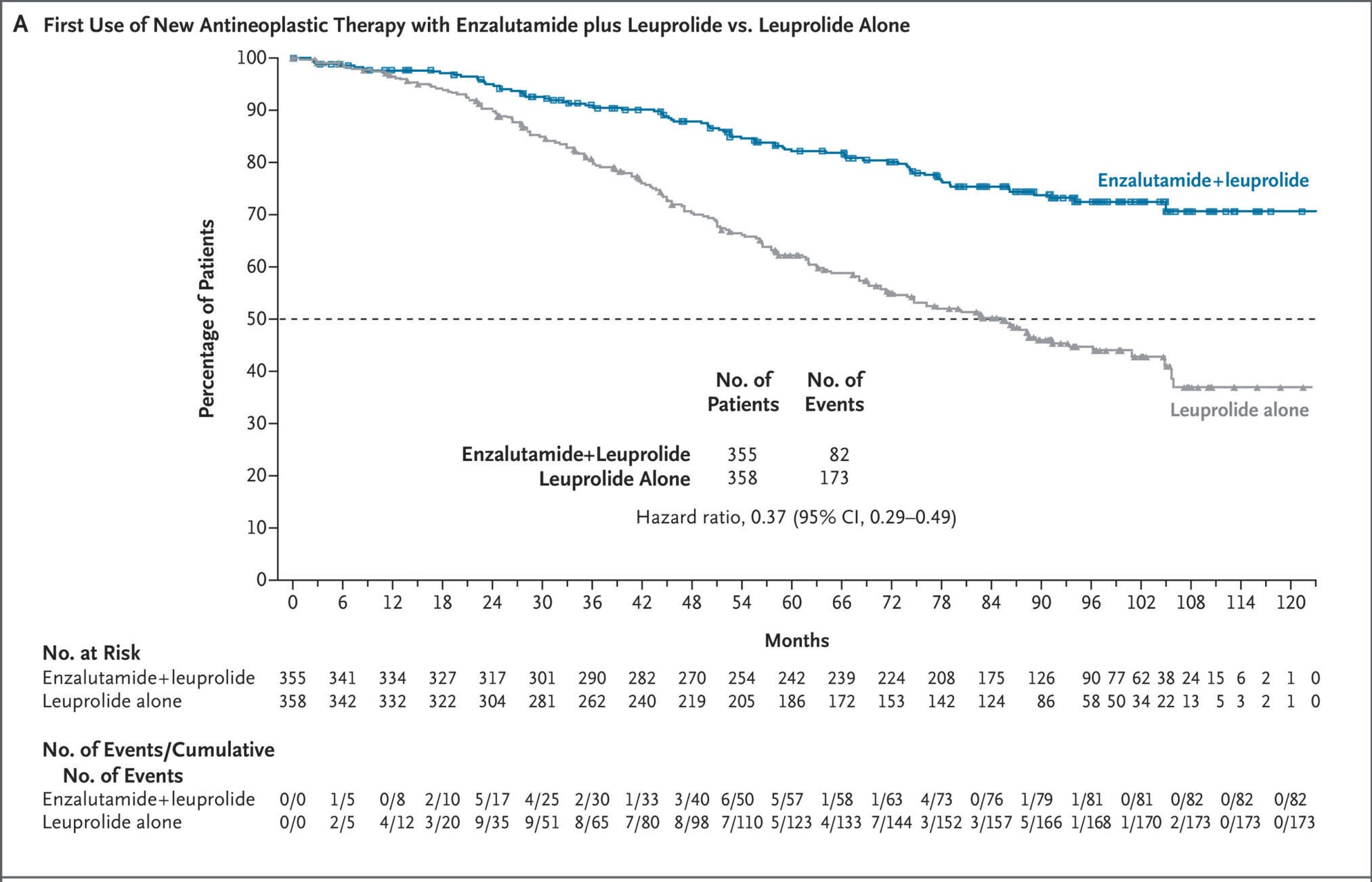
Patients who received the drug combination had not only longer survival but also delay in need for additional cancer treatment. Only 82 men in the combination group required another anticancer treatment, compared with 173 men who received leuprolide alone.


Bone complications, a common and painful problem for men with advanced prostate cancer, also were fewer in number. Just 16 men receiving the combination therapy experienced a major bone event, versus 37 receiving leuprolide alone.
“This current clinical trial shows that intensifying therapy at the right time can become a dramatic difference,” said Dr. Hyung Kim, Urology chair at Cedars-Sinai. “It complements earlier research showing that enzalutamide improves survival in other settings of prostate cancer, and it’s going to change the treatment paradigm of how we treat our patients.”
As with most cancer drugs, the combination was not without adverse effects. Men were fatigued and had hot flashes, and men on the combination therapy experienced more falls and fractures than men on leuprolide alone. Albeit this, the rate of serious treatment-emergent events was low, and there were no fatalities due to the therapy itself.
Enzalutamide alone, even if less burdensome for certain aspects of sex health, did not improve survival when compared with leuprolide. Nevertheless, previous studies suggested that it might still be an option for certain patients who prioritize quality of life over optimal survival.
“This combination not only adds life, but also helps delay the need for stronger drugs or chemotherapy,” added Freedland. “For men who’ve already had radiation or surgery, that can mean extra years of excellent disease control.”
When EMBARK began, doctors relied on traditional imaging like CT and bone scans to see whether or not prostate cancer had spread. Today, advanced PSMA PET-based scans can detect small bits of disease that previous technology would have missed.
Since PSMA PET was not on the market at the beginning of the study, certain men who were categorized as “non-metastatic” could have had unrecognized early spread. Nevertheless, the trial still demonstrated a survival advantage, implying that more potent hormonal blockade will be beneficial even when cancer cannot be seen on scans.
Subsequent studies will likely use PSMA PET to individualize when therapy should start or stop, especially for those whose PSA level rises but whose scans show very minimal disease. Researchers are also looking into whether treatment interruptions—such as the planned interruptions in this study—can reduce side effects without sacrificing control of the cancer.
“This trial is the best of translational medicine,” Dr. Robert Figlin, interim director of Cedars-Sinai Cancer, said. “It brings scientific discovery directly to the bedside and improves outcomes for patients everywhere.”
For recurrent high-risk prostate cancer in men, enzalutamide plus leuprolide may become the new standard of care soon. The therapy offers a clear-cut survival advantage, extends disease-free survival, and reduces serious bone complications without introducing new safety concerns.
Beyond direct clinical benefits, results show the possibility of new technological advances in hormonal therapy to be combined with advanced imaging to find and treat prostate cancer earlier.
For patients, that means more time—and better quality of life—before consenting to aggressive treatments. For researchers, it also introduces new questions about timing, dosing, and how best to adapt therapy to the many different phases of recurrence.
Research findings are available online in the New England Journal of Medicine.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post New drug combo cuts risk of death for men with prostate cancer by 40% appeared first on The Brighter Side of News.