The growing antimicrobial crisis threatens modern medicine, as the world grapples with rising antibiotic resistance and a stagnant pipeline for new antibiotics. Millions of deaths globally are already attributed to resistant infections.
Historically, soil-based actinobacteria have been the cornerstone of antibiotic discovery, producing over 80% of licensed antibiotics. Yet, the oceans, particularly marine actinobacteria, remain largely untapped for their potential to yield novel antimicrobial compounds.
An emerging approach, published in the journal, Frontiers in Microbiology, focuses on inhibiting bacterial virulence rather than killing bacteria outright or halting their growth. This strategy seeks to neutralize bacteria’s ability to cause disease by targeting virulence mechanisms. By leaving pathogens alive but incapable of causing symptoms, this method minimizes selective pressure for resistance.
These antivirulence drugs also offer the advantage of fewer side effects on beneficial bacteria. Natural products have been a rich source of such compounds, including inhibitors of virulence molecule expression, effectors, pilicides, and adhesion blockers.

Enteropathogenic Escherichia coli (EPEC) exemplifies the urgent need for antivirulence therapies. A gram-negative bacterium, EPEC is a leading cause of diarrheal illnesses and high child mortality rates, particularly in developing regions. EPEC infections disrupt intestinal microvilli and epithelial layers, impairing nutrient absorption.
The bacterium’s virulence hinges on its type III secretion system (T3SS), which injects harmful factors into host cells. Among these factors, the translocated intimin receptor (Tir) plays a central role by triggering abnormal actin polymerization. This process forms actin-rich pedestals beneath bacteria, facilitating infection.
EPEC’s antimicrobial resistance compounds the problem. The bacterium exhibits resistance to fluoroquinolones, carbapenems, colistin, and extended-spectrum beta-lactamases. This multifaceted resistance underscores the necessity for innovative solutions that target virulence pathways. By disrupting the Tir-intimin interaction, antivirulence therapies could offer a viable alternative to traditional antibiotics.
To address the crisis, researchers turned to marine actinobacteria from the Arctic Sea. These microorganisms, isolated near Svalbard, hold promise for novel antivirulence compounds. In a recent study, extracts from four marine actinobacteria species underwent rigorous screening for their ability to neutralize EPEC virulence without inhibiting bacterial growth.
Related Stories
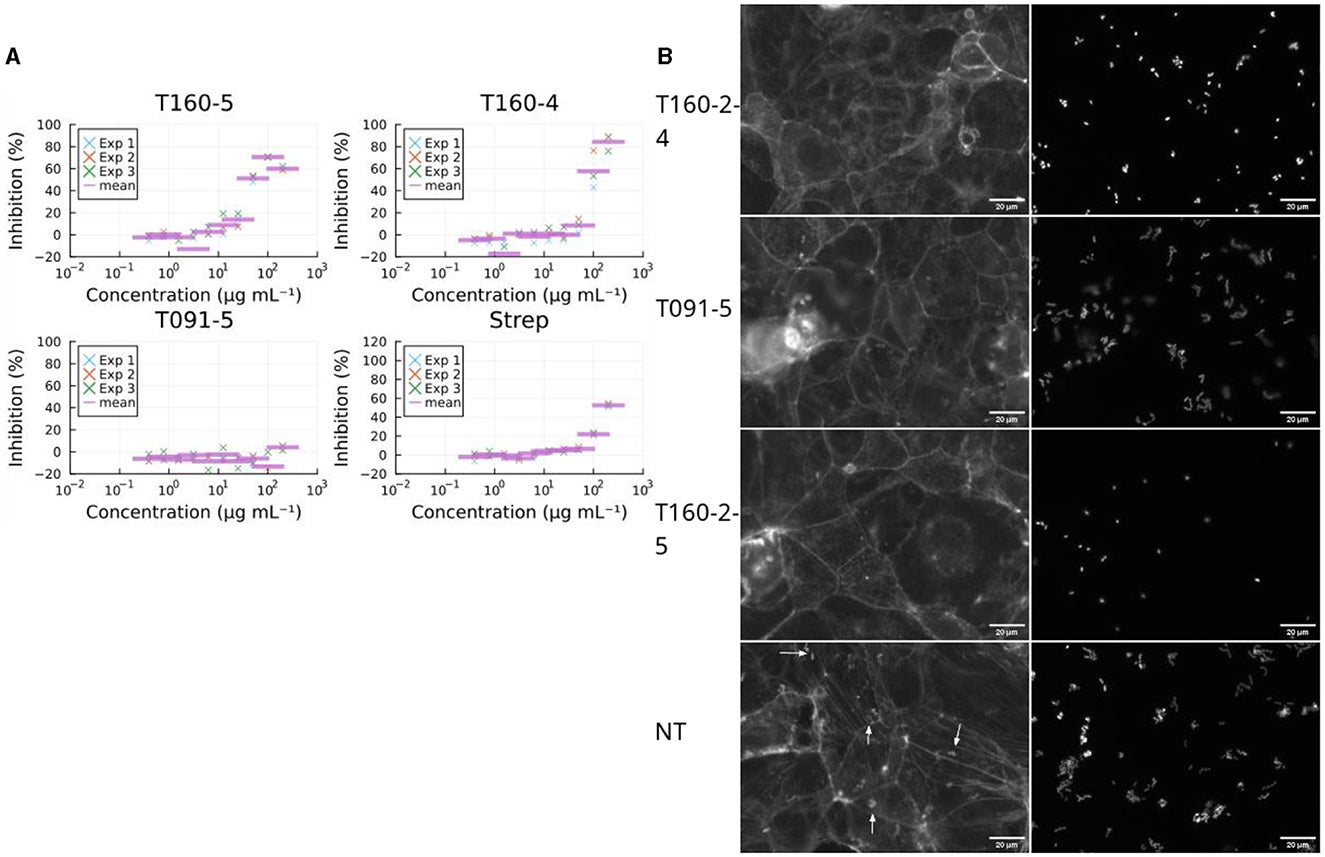
The workflow involved high-throughput screening of bacterial fractions for their effects on three critical activities: translocation of Tir, prevention of actin pedestal formation, and inhibition of EPEC growth.
Bioactive fractions underwent further analysis to identify their mechanisms of action and chemical structures. The assays demonstrated suitability for screening complex mixtures, overcoming challenges posed by pan-assay interference compounds (PAINS) and high concentrations.
The study uncovered compounds with powerful antivirulence properties. A strain from the genus Kocuria yielded a compound that interfered with EPEC-induced actin polymerization without affecting bacterial growth.
Another compound from a Rhodococcus strain exhibited antibacterial activity. These discoveries highlight the potential of marine actinobacteria to address antimicrobial resistance through innovative approaches.
The compound from Kocuria disrupted actin pedestal formation, a key step in EPEC’s infection process. By targeting the interaction between Tir and intimin, it prevented the bacterium from hijacking host cell machinery. Importantly, this compound did not slow bacterial growth, reducing the likelihood of resistance development.
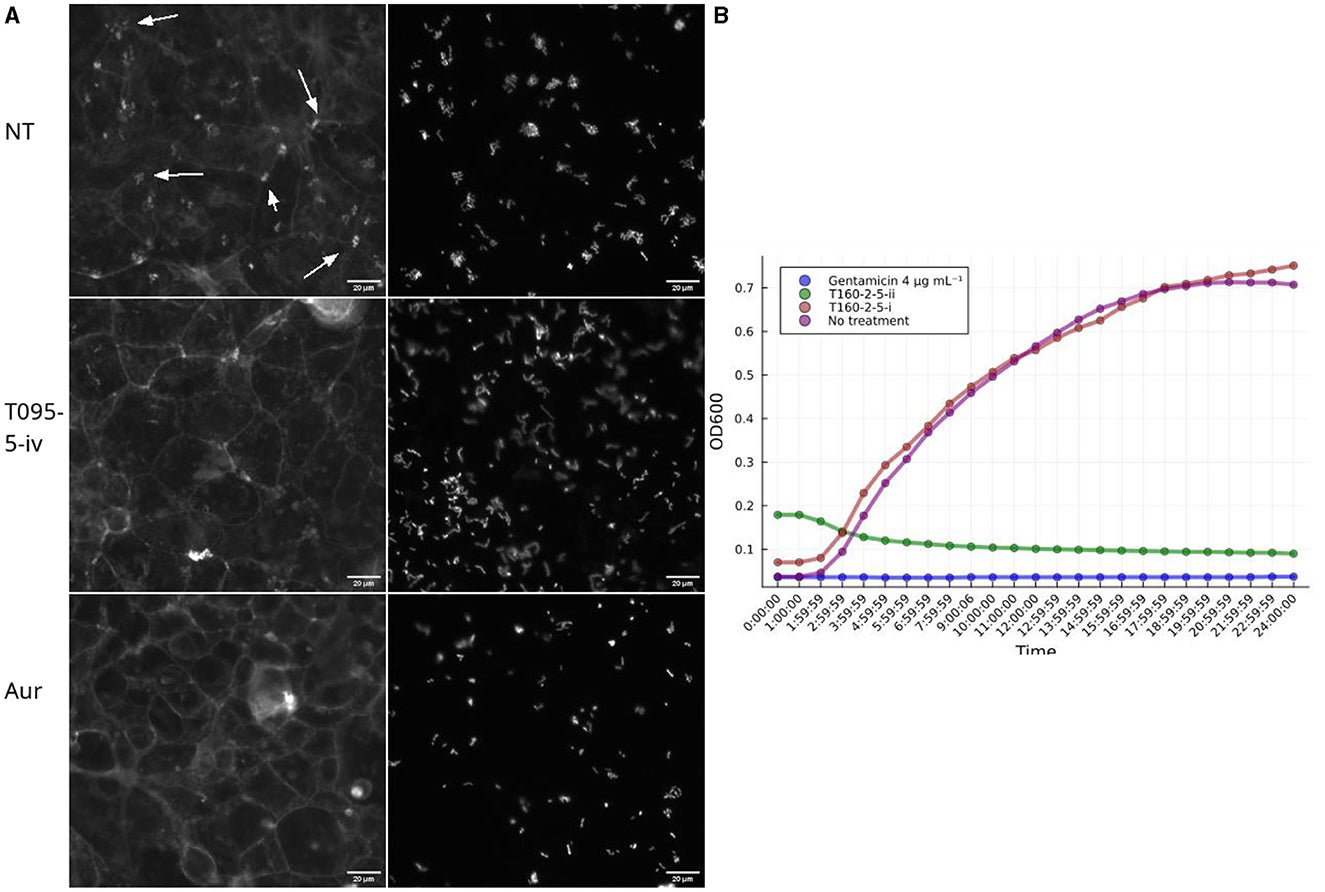
Advanced techniques, such as HPLC-MS-based dereplication, revealed that this compound is likely a phospholipid, a class of molecules crucial to cell metabolism.
“Here we show how advanced screening assays can identify antivirulence and antibacterial metabolites from actinobacteria extracts,” said Dr. Päivi Tammela, a professor at the University of Helsinki. “We discovered a compound that inhibits EPEC virulence without affecting its growth, and a growth-inhibiting compound, both in actinobacteria from the Arctic Ocean.”
These findings pave the way for alternative treatments that mitigate antimicrobial resistance. By targeting virulence rather than bacterial viability, these compounds avoid the pitfalls of conventional antibiotics. The specificity of antivirulence drugs also minimizes disruptions to the microbiome, preserving beneficial bacteria.
The workflow developed in this study offers a blueprint for future research. It integrates isolation, fractionation, and high-throughput screening to identify bioactive compounds from complex microbial mixtures. This approach could accelerate the discovery of novel therapies for resistant pathogens beyond EPEC.
Dr. Tammela emphasized the next steps: “The optimization of culture conditions for compound production and the isolation of sufficient amounts of each compound will allow further investigation of their structures and bioactivities.”
The antimicrobial crisis demands urgent action. Traditional antibiotic development has stagnated, while resistance rates continue to climb. Exploring underutilized environments like the Arctic Ocean offers hope for breakthroughs. Marine actinobacteria, with their unique biochemical capabilities, represent a valuable resource for drug discovery.
Antivirulence therapies hold particular promise as a sustainable solution. By neutralizing pathogens without killing them, these treatments sidestep the evolutionary arms race driving resistance. The compounds identified in this study demonstrate the feasibility of such approaches, providing a foundation for future innovations.
As researchers refine their methodologies and scale up production, these discoveries could transform the fight against resistant infections. The path forward involves collaboration between microbiologists, chemists, and pharmaceutical developers to translate these findings into effective therapies.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Arctic Sea discovery can neutralize bacteria’s ability to cause disease appeared first on The Brighter Side of News.