Researchers at the University of New South Wales Sydney are testing a new way to treat chronic nerve pain by training the brain itself. The approach relies on an interactive game that teaches people to adjust abnormal brain activity linked to persistent pain, without drugs or invasive procedures.
The technology, called PainWaive, was developed by neuroscientists at UNSW Sydney’s NeuroRecovery Research Hub in collaboration with Neuroscience Research Australia. The work is led by Professor Sylvia Gustin and Dr. Negin Hesam-Shariati. Early trial results were recently published in the Journal of Pain.
The research targets neuropathic pain, a form of nerve pain that often resists standard treatments. One example is corneal neuropathic pain, a condition that can develop after eye surgery, chronic dry eye disease, autoimmune disorders, or exposure to harsh light. The pain often feels like burning or stinging and may disrupt sleep, mood, work, and daily activities. Traditional medications and eye treatments rarely provide lasting relief.

Instead of trying to block pain signals with medication, the UNSW team focused on how chronic nerve pain alters communication inside the brain. Their goal was to determine whether teaching people to reshape those signals could reduce pain intensity over time.
PainWaive is built on years of research by Prof. Gustin into how neuropathic pain changes brain activity, particularly in the thalamus, a central relay hub for sensory information.
“The brainwaves of people with neuropathic pain show a distinct pattern: more slow theta waves, fewer alpha waves, and more fast, high beta waves,” Prof. Gustin said.
“We believe these changes interfere with how the thalamus talks to other parts of the brain, especially the sensory motor cortex, which registers pain.”
Those altered rhythms appear to lock the brain into a pain-sensitive state. Prof. Gustin began asking whether a treatment could directly target and rebalance those signals.
“I wondered, can we develop a treatment that directly targets and normalizes these abnormal waves?” she said.

That question led to the development of PainWaive, a home-based EEG neurofeedback system designed to help users shift their brain activity toward healthier patterns through repeated training.
“The first PainWaive trial was designed as a single-case experimental study. Rather than comparing large groups, this format tracks detailed changes within each participant over time. Four adults with long-standing corneal neuropathic pain took part. Each had experienced persistent pain for at least three months and rated their pain at three or higher on a zero-to-10 scale,” Dr. Hesam-Shariati told The Brighter Side of News.
“Participants began the study at the same time but completed different baseline periods ranging from seven to 17 days. This allowed researchers to compare each person’s pain patterns before and after training. Each participant then completed 20 neurofeedback sessions at home over four weeks,” she continued.
Follow-up assessments were conducted immediately after the training period and again five weeks later. Throughout the study, participants completed daily surveys tracking pain severity and how much pain interfered with sleep, work, mood, and daily activity. Measures of anxiety, depression, and sleep quality were also collected.
Each participant received a kit containing an EEG headset and a tablet preloaded with the PainWaive app. During each session, users recorded their brain activity and played short interactive games that responded in real time to changes in their brain waves.

The system focused on three signals recorded from the sensorimotor cortex. Theta waves and high-beta waves, which are often elevated in neuropathic pain, were reduced. The sensorimotor rhythm, which is usually suppressed in pain conditions, was strengthened.
When brain activity moved in the desired direction, the game environment responded. A jellyfish drifted more calmly. A rocket climbed higher. A plane glided more smoothly. Music played in the background to encourage relaxation.
Participants were given suggestions for mental strategies, such as focused breathing, relaxation, or recalling positive memories, to help guide their brain activity. EEG data were uploaded automatically so the research team could monitor progress remotely.
“After just a couple of Zoom sessions, participants were able to run the treatment entirely on their own,” Dr. Hesam-Shariati said. “Participants felt empowered to manage their pain in their own environment. That’s a huge part of what makes this special.”
After four weeks of training, three of the four participants showed statistically significant reductions in pain severity, pain interference, or both. The greatest improvements appeared toward the end of the treatment period and during follow-up.
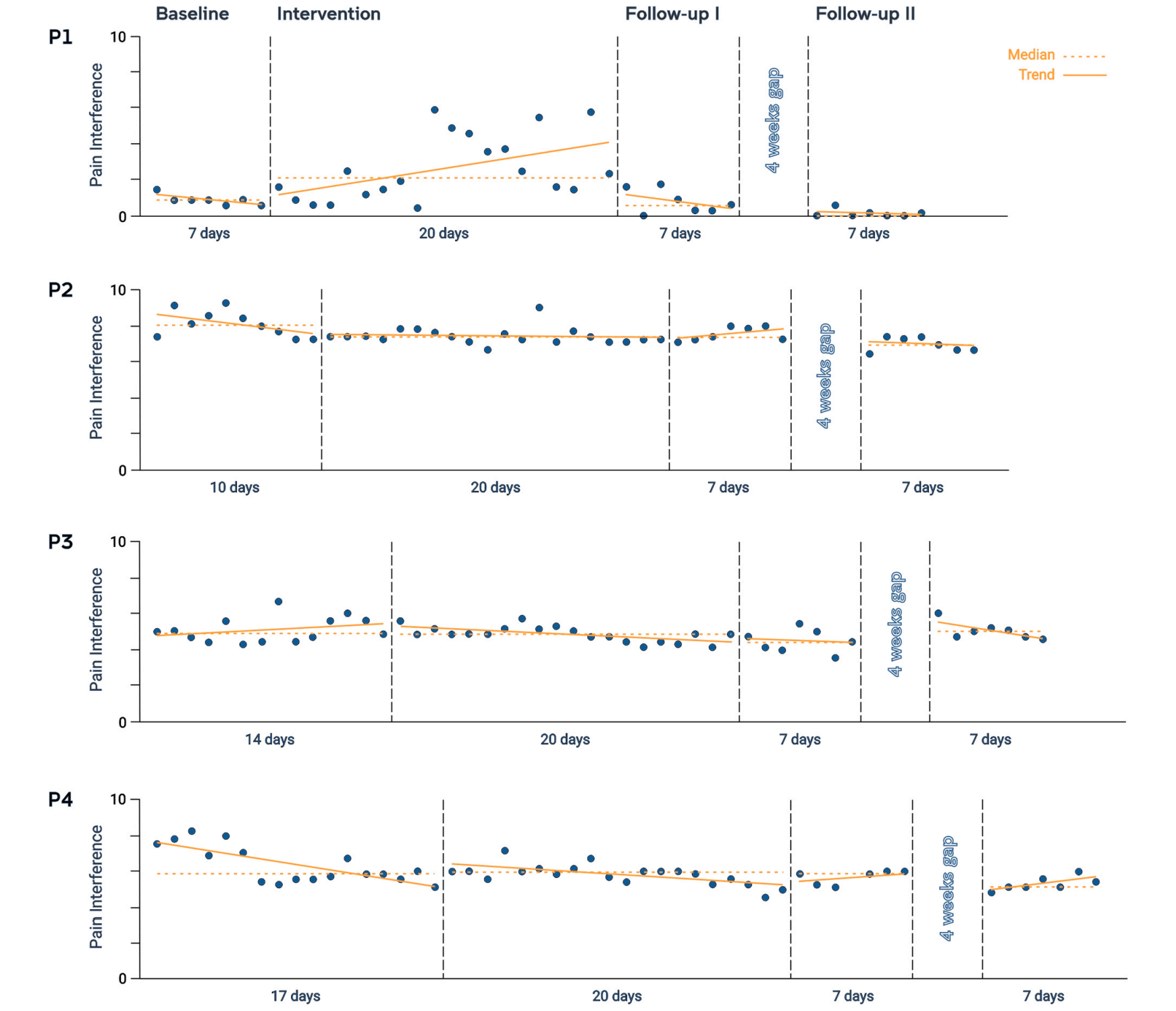
Overall, the level of pain relief observed in those three participants was comparable to, and in some cases greater than, what is typically reported with opioid treatment.
“Restrictions in the study’s size, design and duration limit our ability to generalize the findings or rule out placebo effects,” Dr. Hesam-Shariati said.
“But the results we’ve seen are exciting and give us confidence to move to the next stage and our larger trial.”
The researchers caution that although statistical improvements were detected, changes were modest for some participants. Not all pain reductions reached levels considered clinically meaningful. One participant experienced little change, highlighting the need to understand who benefits most from this approach.
Participants consistently reported positive experiences using the system. Many described the sessions as calming and compared them to meditation. All rated the system as easy to use, with usability scores above 85 out of 100.
The team also focused heavily on affordability and comfort. Commercial EEG systems were either too expensive or lacked sufficient signal quality. As a result, the researchers built nearly every component themselves.
“Everything except the open-source EEG board was built in-house,” Dr. Hesam-Shariati said. “And soon, even that will be replaced by a custom-designed board.”
By using 3D printing and simplified electronics, the team reduced the headset cost to about $300. Comparable systems can cost between $1,000 and $20,000.
“We’ve worked closely with patients to ensure the headset is lightweight, comfortable, and user-friendly,” Prof. Gustin said.
The PainWaive study offers early evidence that directly training brain activity may help relieve chronic nerve pain when traditional treatments fail. If confirmed in larger trials, this approach could expand pain management options without relying on medications or invasive procedures.
For patients with limited access to specialist care, a home-based system could reduce barriers to treatment and increase independence. The technology may also help researchers better understand how pain-related brain patterns differ between individuals, opening the door to more personalized therapies.
Beyond corneal neuropathic pain, the findings may inform future research into other chronic pain conditions linked to abnormal brain rhythms. The work suggests that targeting the brain’s electrical patterns could become a meaningful complement to existing treatments rather than a replacement.
Research findings are available online in the Journal of Pain.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Brain training game shows early promise for treating chronic pain without drugs appeared first on The Brighter Side of News.