New research published in the journal Disease Models & Mechanisms offers insights into how glial cells — the supportive cells of the nervous system — may influence the progression of Alzheimer’s disease. Using fruit flies as a model, researchers found that disrupted glucose metabolism in glial cells can worsen inflammation and damage to neurons triggered by the accumulation of tau protein. Enhancing glucose uptake in specific glial cells significantly reduced these harmful effects, highlighting a potential new therapeutic direction for slowing neurodegenerative processes.
Alzheimer’s disease is marked by the abnormal buildup of tau protein inside brain cells, along with brain inflammation and reduced glucose metabolism. While these features are well known, how they relate to each other has remained uncertain. In particular, scientists have been curious about the role of glial cells, which help maintain a healthy environment for neurons. These cells are also involved in immune responses in the brain and have been implicated in the progression of Alzheimer’s and other neurodegenerative conditions.
Given their key role in energy metabolism and inflammation, researchers from Tokyo Metropolitan University aimed to better understand how tau protein accumulation affects glial function, and whether changes in glial metabolism contribute to neurodegeneration.
To explore this, the researchers turned to the fruit fly Drosophila melanogaster, a widely used model organism in neuroscience. They genetically engineered the flies to express human tau protein in the retina, which includes both neurons and glial cells. This model allowed the researchers to observe signs of neurodegeneration, such as damage to photoreceptor cells and the appearance of abnormal, dense inclusions. They also noticed swelling in nearby brain regions, suggesting that inflammation was taking place. Microscopic analysis confirmed that the inclusions were formed by overactive glial cells attempting to clean up cellular debris — a process that can become damaging when uncontrolled.
The researchers then looked more closely at whether tau expression in the retina was affecting glucose metabolism. They introduced a human gene known as GLUT3, which enhances glucose uptake, into the flies. Interestingly, adding GLUT3 to the tau-expressing retina did not reduce the amount of tau protein, but it did significantly reduce inflammation, inclusion formation, and photoreceptor damage. This suggested that supporting glucose metabolism in glial cells could counteract the damaging effects of tau buildup.
To test whether the protective effects were specific to glial cells, the researchers expressed GLUT3 only in pigment glial cells, a type of glia that supports photoreceptor neurons in the fly retina. These pigment glia are known to help neurons by supplying nutrients and removing waste. When GLUT3 was expressed in these glial cells, the researchers again observed less swelling, fewer signs of inflammation, and improved survival of photoreceptor cells. In contrast, expressing GLUT3 in neurons did not reduce the damage, suggesting that the beneficial effects stemmed from changes in glial cell metabolism.
In addition to structural damage, the researchers examined gene expression and found that tau expression led to increased levels of antimicrobial peptides, which are typically produced during immune responses. These peptides are part of the inflammatory signaling pathways in flies, and their elevated levels further supported the idea that glial cells were becoming pathologically active in response to tau. However, when GLUT3 was expressed in glial cells, the levels of these inflammatory markers dropped, reinforcing the link between glucose metabolism and glial activation.
Interestingly, the protective effects of GLUT3 did not depend on lowering the tau protein itself or preventing its phosphorylation, a process associated with toxicity in Alzheimer’s disease. This suggests that glucose metabolism in glial cells acts downstream of tau accumulation — meaning that while the tau buildup triggers the problem, the glial response, shaped by metabolic capacity, may determine how much damage occurs.
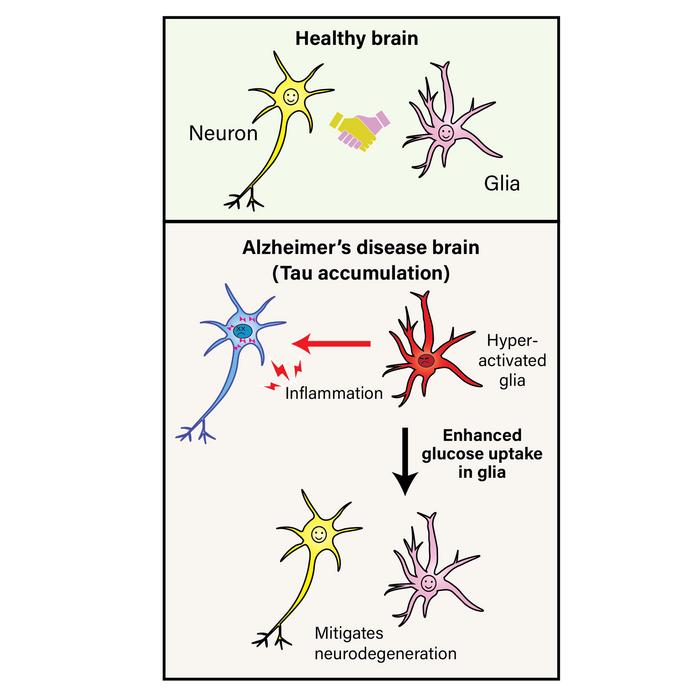
While the study was conducted in fruit flies, the findings point toward a possible role for glial metabolism in human neurodegenerative diseases. In the human brain, glial cells such as astrocytes and microglia are essential for maintaining the health of neurons. These cells are also known to become reactive and inflammatory in Alzheimer’s disease, which can lead to further neuronal damage. By showing that glucose metabolism can modulate this response, the study suggests that boosting energy availability in glial cells could help reduce inflammation and protect neurons — even when the underlying causes of the disease, such as tau buildup, remain.
The study has several strengths, including its detailed analysis of retinal structure, protein levels, and gene expression, as well as its use of both neuron- and glia-specific gene expression to pinpoint where the protective effects were occurring. However, as with all animal studies, there are limitations when it comes to applying the findings directly to humans. Fruit flies are useful models for basic biological processes, but their brains are much simpler than those of mammals. Additional research in mammals, including mice, will be necessary to confirm whether enhancing glucose metabolism in glial cells has similar protective effects in more complex brains.
Future research could also explore whether similar metabolic interventions might help in other neurodegenerative diseases that involve inflammation, such as Parkinson’s or amyotrophic lateral sclerosis. The authors also point out that further studies using advanced techniques like metabolic profiling and transcriptomics could help clarify the molecular pathways through which glucose affects glial behavior. Understanding these pathways could lead to new drugs that modulate glial metabolism or inflammation more precisely.
The study, “Glucose uptake in pigment glia suppresses Tau-induced inflammation and photoreceptor degeneration,” was authored by Mikiko Oka, Sho Nakajima, Emiko Suzuki, Shinya Yamamoto, and Kanae Ando.