The battle against rectal cancer has entered an extraordinary new phase with the advent of immunotherapy, offering hope to patients and redefining treatment paradigms.
A groundbreaking clinical trial conducted at Memorial Sloan Kettering Cancer Center (MSK) has demonstrated unparalleled success, achieving a 100% remission rate among participants. This trial leverages the immune system to fight cancer in patients with specific genetic mutations, bypassing the life-altering side effects of conventional treatments.
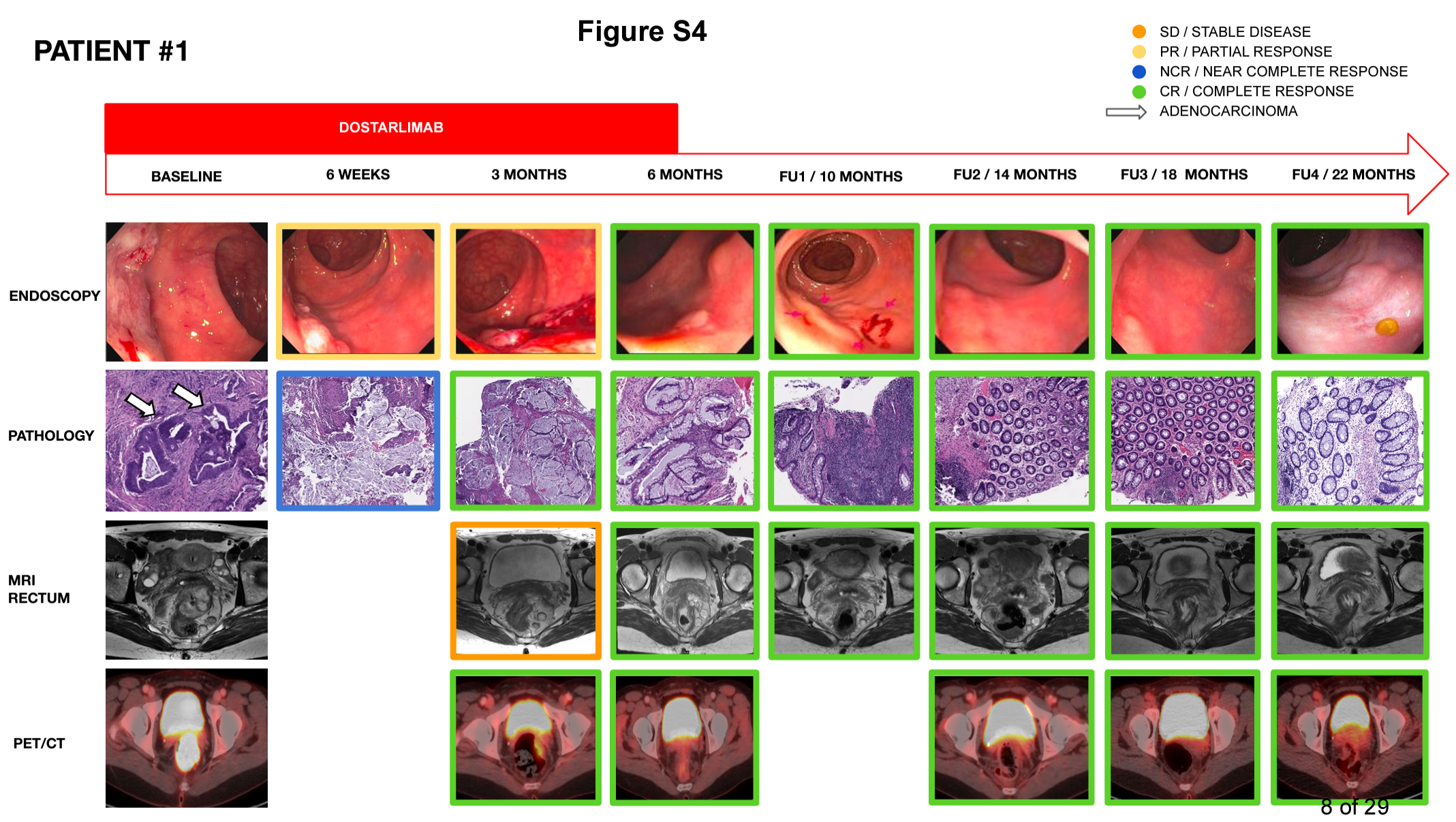
In this transformative trial, 42 patients with mismatch repair-deficient (MMRd) or microsatellite instability-high (MSI) rectal tumors were treated with dostarlimab, an immunotherapy agent marketed as Jemperli. Administered every three weeks over six months, this treatment eliminated the need for surgery, radiation, or chemotherapy.

Dr. Andrea Cercek, a gastrointestinal oncologist at MSK and one of the trial’s lead researchers, reports, “So far, 42 people have completed treatment, and all of them have no evidence of disease. Side effects were mild and well-tolerated.”
The success of this trial was celebrated at the 2024 American Society of Clinical Oncology (ASCO) conference, marking a milestone in cancer research. Dr. Cercek revealed that the original participants have been cancer-free for up to four years, and the protocol has already influenced global clinical practices.
In the United States, the National Comprehensive Cancer Network updated its guidelines to incorporate this approach, prompting insurers to cover the treatment.
The FDA recognized dostarlimab’s transformative potential by granting it a “Breakthrough Therapy Designation” in December 2024. This expedited approval process underscores the substantial improvement this treatment offers over traditional methods, which often involve invasive surgeries and toxic therapies.
Related Stories
Dostarlimab functions as a checkpoint inhibitor, releasing immune cells to recognize and attack cancer. MMRd tumors, which comprise 5–10% of rectal cancers, lack the ability to repair certain genetic mutations, making them especially vulnerable to this approach.
Dr. Luis Diaz, co-lead of the trial and a member of the White House’s National Cancer Advisory Board, explains, “When the brakes are taken off immune cells, MMRd cells look especially strange because they have so many mutations. The immune cells attack with much more force.”
This strategy builds on Dr. Diaz’s earlier successes with immunotherapy in metastatic colorectal cancer. He notes, “We thought, ‘Let’s try it before cancer metastasizes as a first line of treatment.’” The results exceeded expectations, as tumors disappeared completely in all participants, eliminating the need for additional interventions.
The implications of this trial extend far beyond remission statistics. Traditional treatments for rectal cancer, including chemotherapy, radiation, and surgery, often result in profound physical and psychological consequences. These include bowel and bladder dysfunction, incontinence, infertility, and diminished sexual health. As Dr. Cercek notes, “Our first duty is to save our patients’ lives, but we must also consider their quality of life.”
Sascha Roth, the first participant in the trial, exemplifies the human impact of this groundbreaking approach. Diagnosed with stage II rectal cancer at 38, she was initially scheduled for weeks of radiation therapy. After six months of immunotherapy, her tests showed no trace of cancer. “I was stunned and ecstatic,” Roth recalls. “This treatment spared me from life-altering side effects. It was like a dream.”
Her story is not unique. Three participants have since become parents, an outcome nearly impossible with traditional treatments. “It’s incredibly rewarding to see patients preserve their fertility and normal body functions,” says Dr. Cercek.
The MSK trial has catalyzed global interest and adoption of immunotherapy for rectal cancer. Letters of gratitude have poured in from around the world, and oncologists are incorporating this approach into their practices.
Dr. Diaz believes this success is only the beginning. His team is exploring the potential of immunotherapy for other MMRd cancers, including gastric, prostate, and pancreatic tumors. “We are investigating whether this method can help other cancers where treatments are often life-altering,” he says.
In addition to benefiting cancer patients, the trial underscores the importance of genetic testing. All participants in the MSK study had their tumors tested for MMRd or MSI status, a prerequisite for determining eligibility. Dr. Diaz emphasizes, “Our message is clear: Get tested. If your tumor is MMRd, immunotherapy might be the best option for you.”
This approach is particularly significant for younger patients, who are increasingly affected by rectal cancer. MSK’s Center for Young Onset Colorectal and Gastrointestinal Cancer, co-led by Dr. Cercek, focuses on the unique needs of these individuals. “We are seeing more young people with rectal cancer,” she says. “Immunotherapy offers them new hope.”
As the trial continues, its impact is undeniable. Patients like Roth have not only regained their health but also their confidence and optimism. Today, Roth runs a thriving business and advocates for cancer patients, sharing her story to inspire others.
“MSK’s research is years ahead of where most hospitals are,” she says. “I’m living proof of what’s possible.”
With no reported recurrences or severe side effects, the trial has set a new benchmark for cancer treatment. Dr. Diaz envisions a future where immunotherapy becomes a standard approach for suitable candidates, transforming lives without the collateral damage of traditional treatments.
The success of the MSK trial signals a paradigm shift in oncology. By harnessing the power of the immune system, researchers have not only achieved unprecedented remission rates but also preserved the dignity and quality of life for patients. This trial exemplifies the promise of precision medicine—targeted, effective, and patient-centered care.
As researchers delve deeper into the potential of immunotherapy, the horizon looks brighter for countless individuals. The story of this trial is not just about defeating cancer; it’s about rewriting what’s possible in medicine.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post FDA approved drug achieves 100% remission for rectal cancer appeared first on The Brighter Side of News.