A new clinical study, published in the journal JAMA, has revealed a powerful tool in the fight against teen and young adult nicotine addiction: a twice-daily pill originally meant to help adults stop smoking. When paired with counseling, this treatment dramatically increased the odds of quitting vaping—offering the first evidence-based solution specifically tested in young people.
In a randomized trial funded by the National Institutes of Health, researchers at Massachusetts General Hospital tested the effects of varenicline, a prescription drug already approved by the FDA to help adults stop smoking. Led by Dr. A. Eden Evins, the team found that people aged 16 to 25 who took the drug were more than three times as likely to quit vaping compared to those who received only therapy or text-based support.
“Vaping is extremely popular among kids, and we know that this early nicotine exposure can make drugs like cocaine more addictive down the line, yet ours is the first treatment study to look at this vulnerable population,” said Dr. Evins, who directs the Center for Addiction Medicine at MGH. “We wanted to help teens and young adults quit, and we found that prescribing varenicline is the best way to do that.”

Varenicline works by blocking the effects of nicotine in the brain. It lowers cravings and reduces the pleasure of using nicotine. The drug has shown promise in adults who want to stop smoking, but results in youth have been unclear—until now.
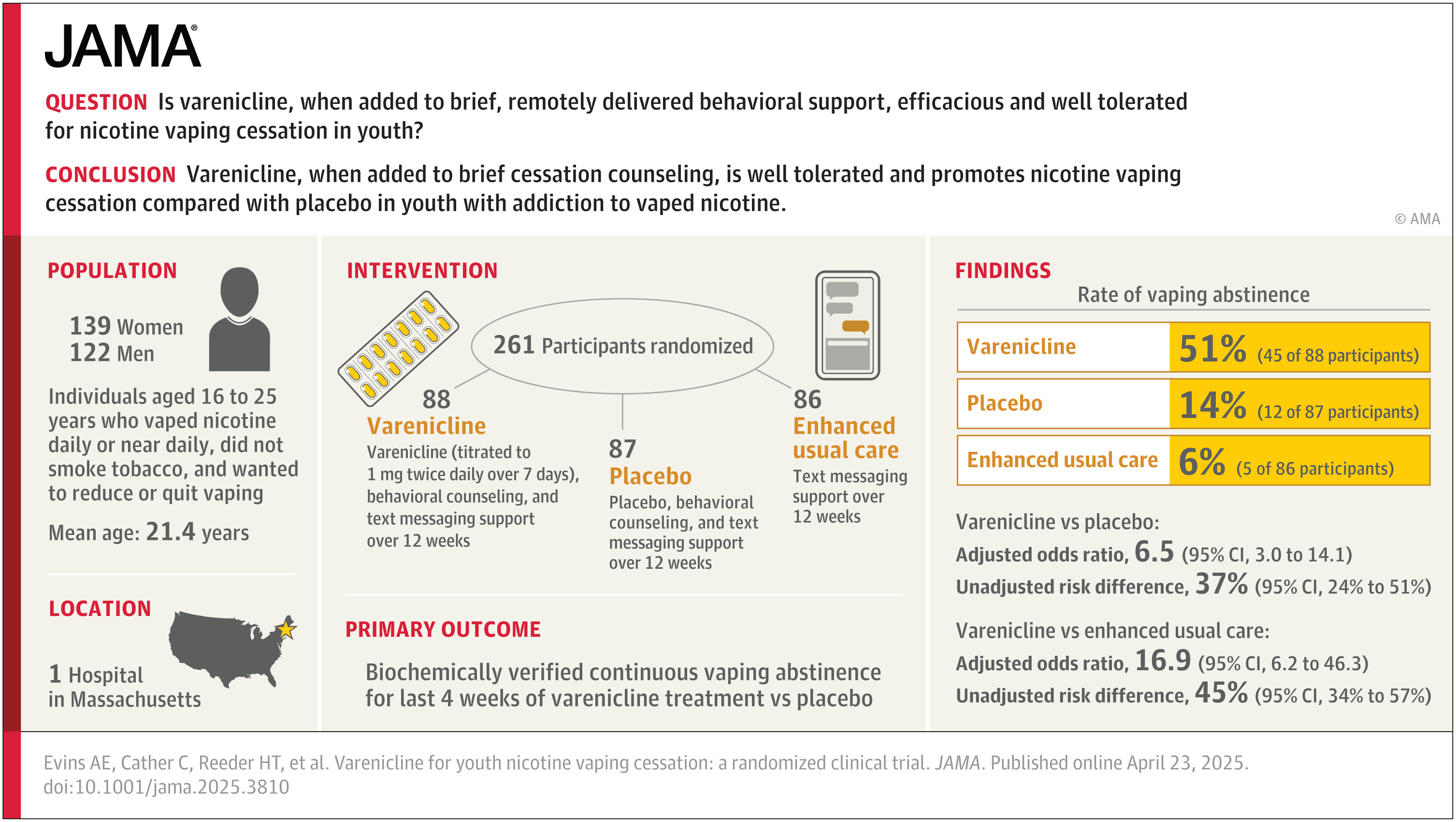
The team recruited 261 teens and young adults aged 16 to 25, all of whom vaped nicotine nearly every day. None were regular cigarette smokers. Participants were randomly assigned to one of three groups for a 12-week treatment period.
The first group took varenicline, attended weekly 20-minute behavioral counseling sessions, and enrolled in a free, text-based quit program called “This is Quitting.” The second group followed the same plan but took a placebo instead of varenicline. The third group used only the text service.
Related Stories
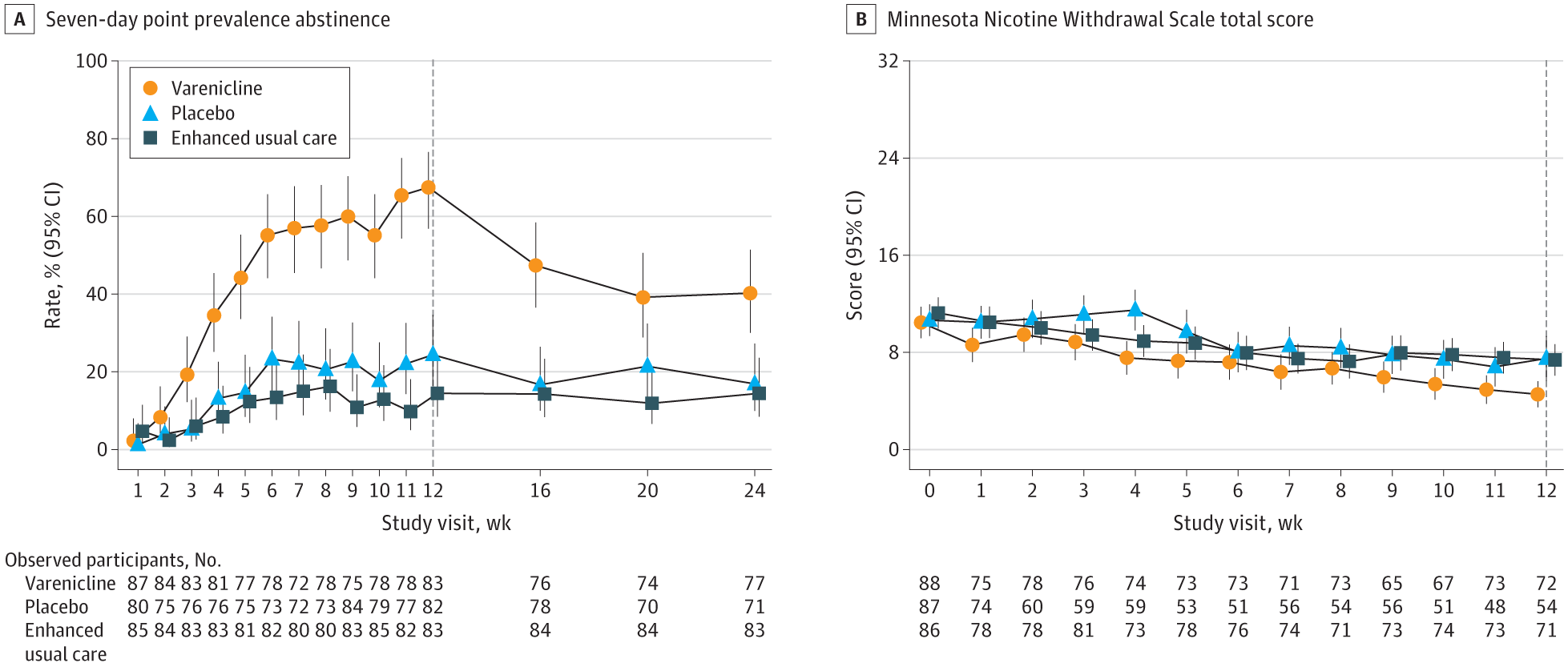
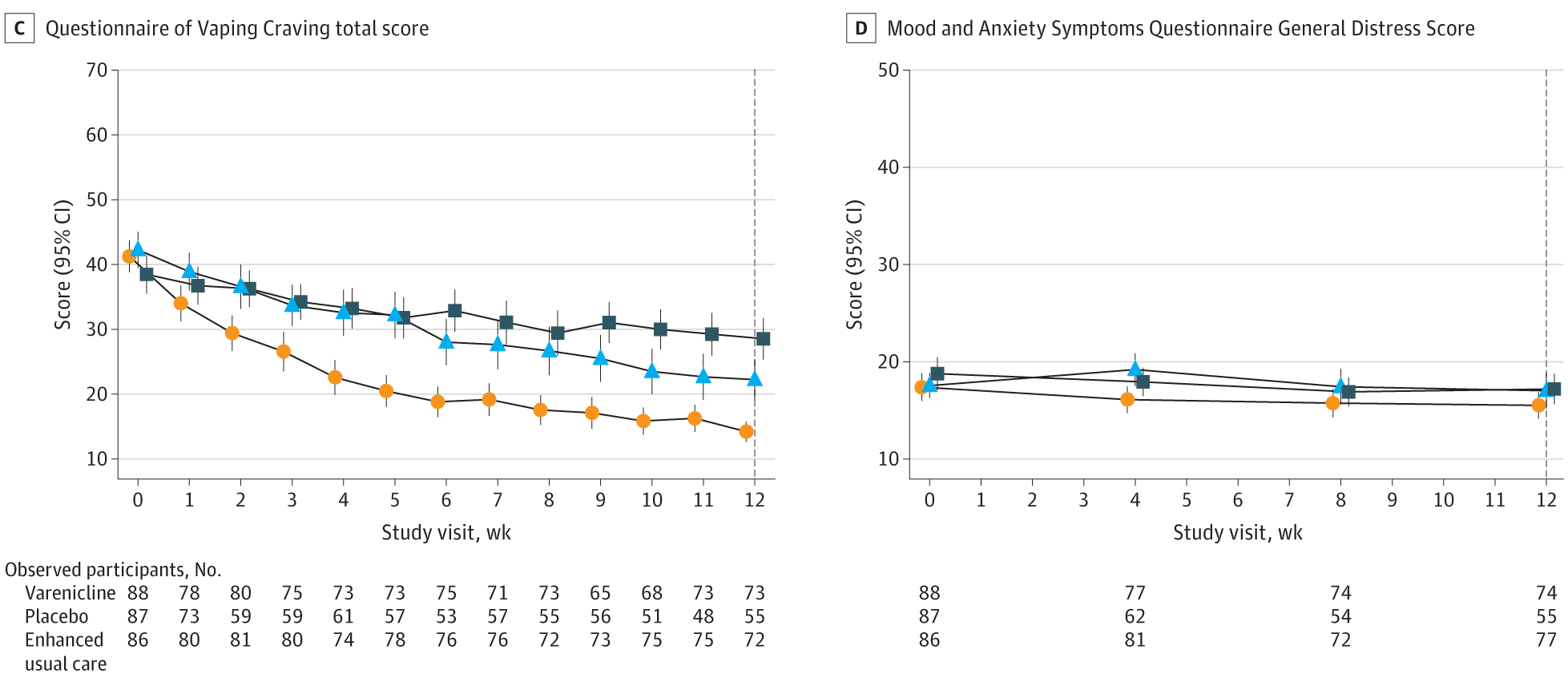
Each participant reported their vaping status weekly, and their claims were confirmed using cotinine saliva tests—a reliable marker of nicotine use. The results were striking. After 12 weeks, 51% of those who took varenicline had stopped vaping. In contrast, only 14% of those who took the placebo and just 6% of those in the text-only group had quit.
Three months after treatment ended, nearly 28% of the varenicline users were still vape-free. That number dropped to 7% in the placebo group and 4% among those using only the text program.
“Not only was varenicline effective in this age group—it was safe. Crucially, we didn’t see any participants that quit vaping turn to cigarettes,” said Dr. Randi Schuster, founding director of the Center for School Behavioral Health at MGH.
Vaping rates among teens and young adults have climbed sharply in recent years. In 2023, nearly 1 in 4 young adults vaped. By 2024, about 8% of high school students reported vaping. Unlike cigarettes, vapes are easier to hide and use in public. But they come with serious risks: nicotine addiction, lung damage, and exposure to carcinogens and heavy metals.
The return of nicotine addiction in this age group has reversed decades of progress in tobacco control. Many teens who vape want to stop but struggle without help. Until now, there has been little research into what treatments actually work for this age group.
Because varenicline is already approved for adult smoking cessation, it can legally be prescribed to people as young as 16. That makes it immediately available to many who need it most.
The drug was well tolerated in the trial, with only three participants dropping out due to side effects—two in the varenicline group and one in the placebo group. And importantly, quitting vaping did not lead to a switch to cigarettes.
The study’s success is a breakthrough, not just for researchers but for public health. It’s the first trial to prove that a medication can help young people quit vaping when paired with counseling and digital support.
The study’s authors stress the need for continued research. They hope to test this treatment in younger teens, as well as in those who vape and smoke tobacco. More options may emerge in the future, but for now, varenicline stands out as a promising tool.
These findings come at a critical time. With youth vaping rates high and nicotine’s effects on the brain well-documented, offering a proven path to quit is essential.
“Vaping is extremely popular among kids, and we know that this early nicotine exposure can make drugs like cocaine more addictive down the line,” Dr. Evins said. “We wanted to help teens and young adults quit, and we found that prescribing varenicline is the best way to do that.”
The message from the study is clear: quitting vaping is possible, and some tools work much better than others. Behavioral therapy alone helped some people, and a text-based support program gave users encouragement. But when these supports were paired with medication, success rates soared.
Many young people are eager to quit vaping but lack the right resources. Now, thanks to this study, doctors have a stronger case for prescribing a treatment that works. And teens and young adults who want to quit have something they didn’t have before—hope backed by science.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post FDA-approved drug triples vaping quit rates in teens and young adults appeared first on The Brighter Side of News.