Advancements in genetics and neuroimaging are unraveling how our DNA shapes the structure and function of the brain. Recent research, including one of the largest studies of its kind, sheds light on genetic factors influencing deep brain regions responsible for memory, motor control, and even psychiatric conditions.
This global effort is not just about mapping genetic associations but also understanding the intricate mechanisms behind brain development and disorders.
A collaborative study led by the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) consortium and published in the journal Nature Genetics involved 189 researchers from 45 countries. By analyzing DNA samples and magnetic resonance imaging (MRI) data from 74,898 participants, the team performed genome-wide association studies (GWAS) to identify genetic variations linked to brain volume.
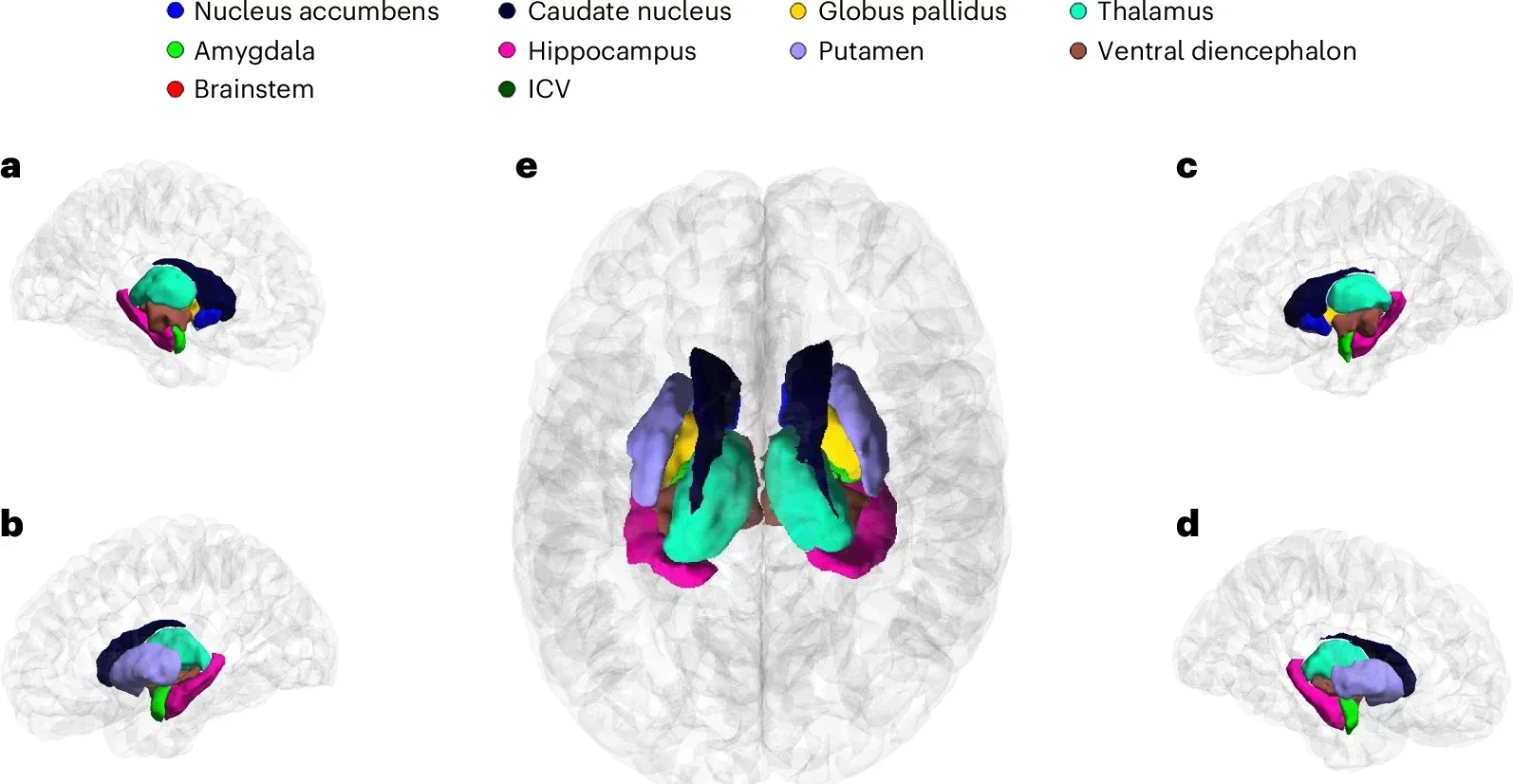
This analysis revealed 254 independent genetic loci associated with key subcortical regions such as the brainstem, hippocampus, and amygdala. These loci explained up to 35% of the variance in intracranial and subcortical brain volumes.
Paul M. Thompson, PhD, a principal investigator for ENIGMA, highlighted the significance of this work. “By conducting this research all over the world, we’re beginning to home in on what has been called ‘the genetic essence of humanity,’” he said. These findings provide a roadmap for understanding how genes influence brain structure and function.
The study uncovered genetic links to specific subcortical regions, which play crucial roles in learning, memory, motor skills, and emotional regulation. Among the findings, the brainstem showed the highest number of independent genetic associations, while the amygdala had the fewest.
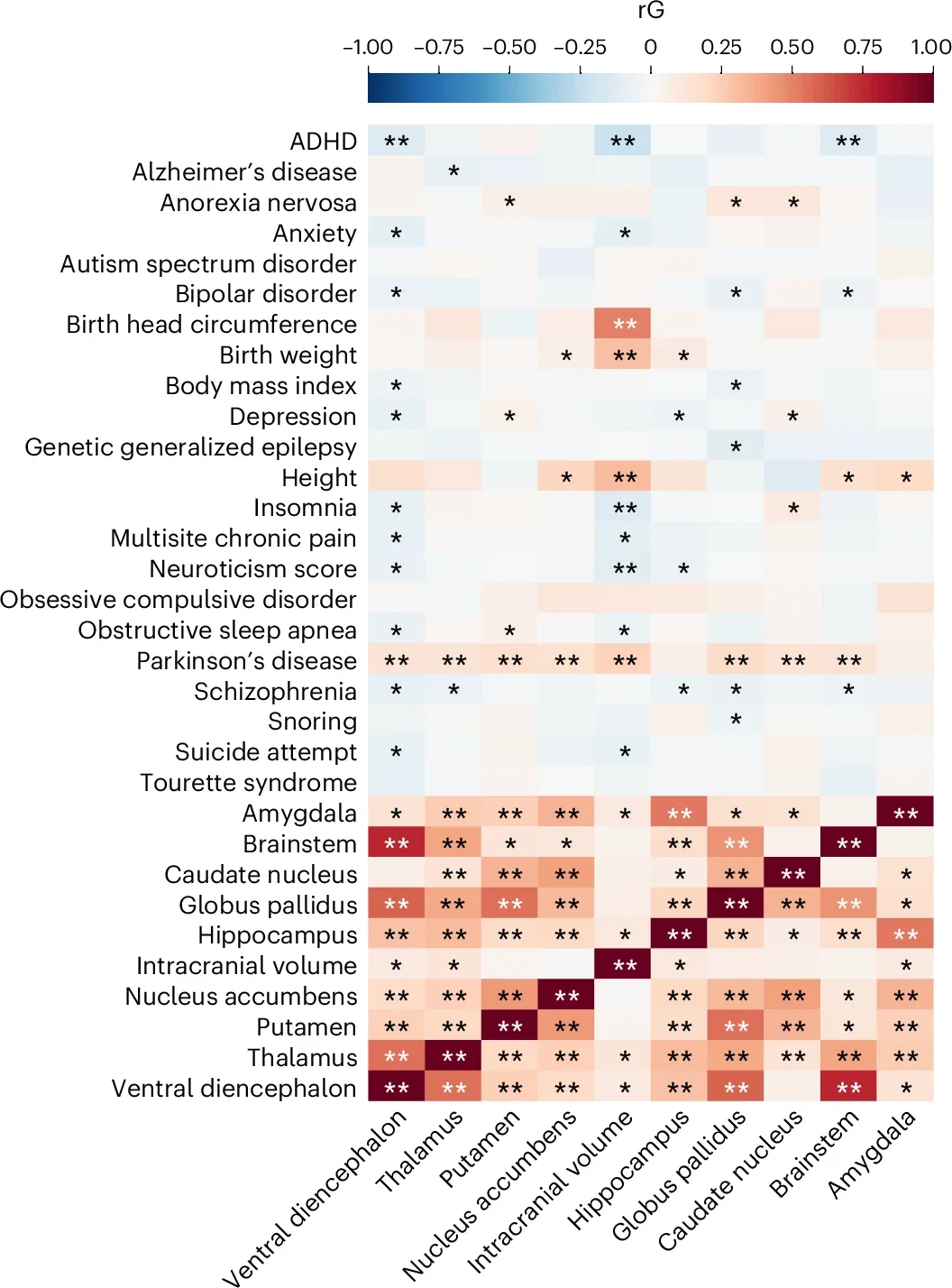
Notably, the genes CRHR1, MAPT, and ZNF786 were associated with subcortical brain volumes, supporting their roles in intracellular signaling and brain aging processes such as tau pathology and vascular resistance.
Functional annotation and gene prioritization using tools like MAGMA and transcriptome-wide association studies (TWAS) further refined the understanding of these genetic influences. For instance, the forkhead box O3 (FOXO3) gene was linked to multiple brain structures, highlighting its broader influence on brain morphology.
Related Stories
Genes from the WNT family were also identified, connecting them to brainstem and ventral diencephalon volumes. These findings suggest potential targets for addressing brain disorders tied to structural variations.
To bolster these findings, researchers integrated single-cell RNA sequencing data with GWAS results. This integration identified specific cell types, such as dopaminergic neurons and astrocyte-like cells, as contributors to brain volume variation. These cell types were linked to critical developmental processes, providing a glimpse into how genetic variations manifest in structural differences during early brain development.
The genetic underpinnings of subcortical brain volumes extend beyond structural analysis to implications for neuropsychiatric and neurological disorders.
Subcortical structures are frequently implicated in conditions like Parkinson’s disease, attention-deficit/hyperactivity disorder (ADHD), and other developmental and psychiatric disorders. For example, the basal ganglia, a region linked to motor control, showed associations with genetic variants implicated in Parkinson’s disease.
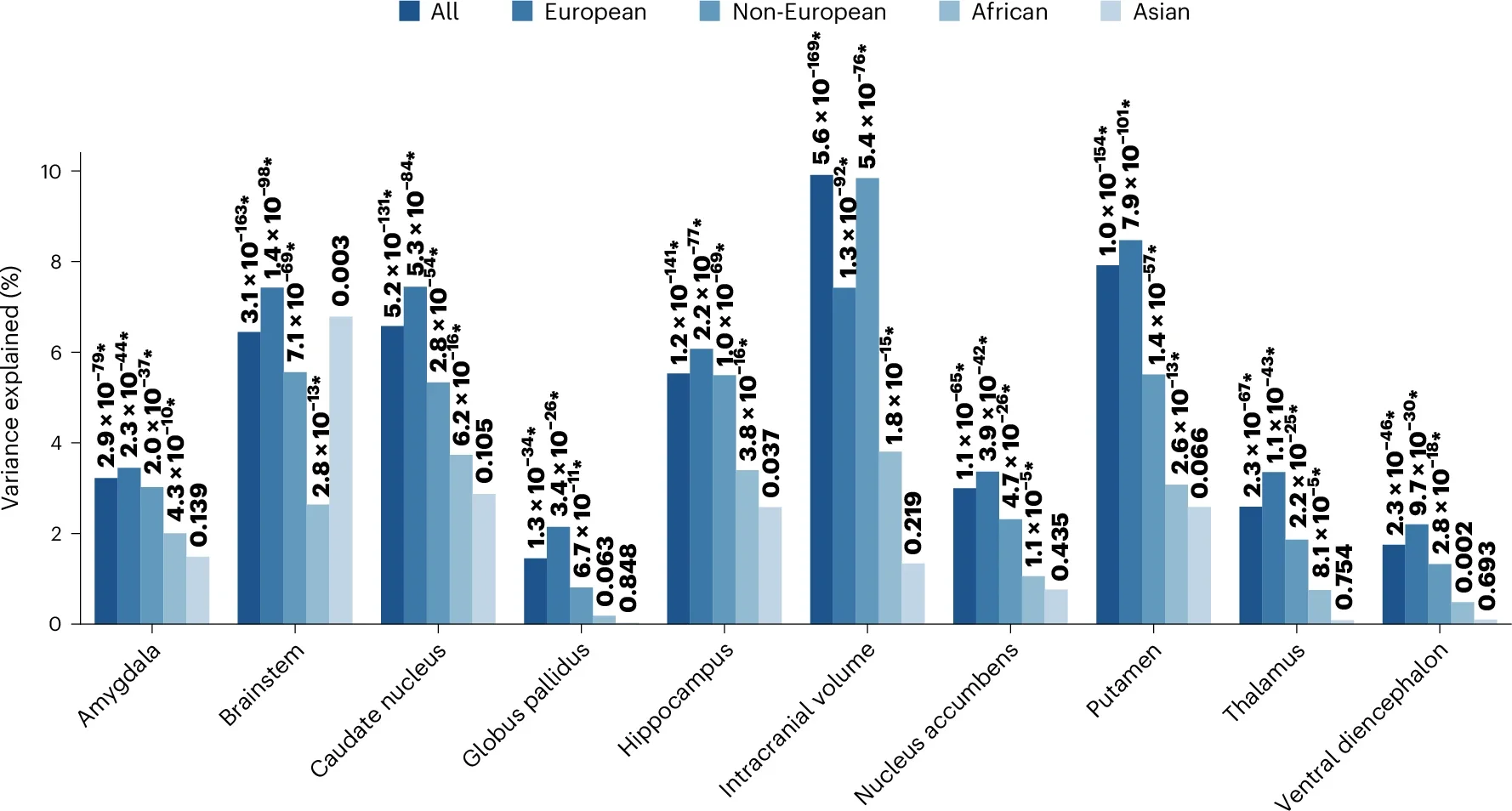
Polygenic scores derived from the GWAS results demonstrated predictive power across diverse populations. These scores explained up to 8.5% of variance in brain volumes among European ancestry participants and up to 9.8% in non-European groups. This cross-ancestral consistency underscores the robustness of genetic associations and their potential utility in understanding disease mechanisms across populations.
Miguel Rentería, PhD, an associate professor of computational neurogenomics, emphasized the broader implications: “Our findings suggest that genetic influences that underpin individual differences in brain structure may be fundamental to understanding the underlying causes of brain-related disorders.”
Interestingly, the study also explored gene-environment interactions, identifying potential influences of lifestyle factors on brain volume. Although genetic predisposition plays a significant role, external factors like diet, exercise, and exposure to environmental toxins may modulate how these genetic factors are expressed.
While this study provides critical insights, it remains correlational, necessitating further research to establish causal links. The integration of single-cell RNA sequencing with GWAS data offers a promising direction. This approach identified cell types like dopaminergic neurons and astrocyte-like cells as key players in brain volume variation. Understanding the specific roles of these cells in genetic pathways could inform targeted interventions.
Furthermore, the research highlights the importance of diverse datasets. By including data from initiatives like the UK Biobank, CHARGE, and the Adolescent Brain Cognitive Development (ABCD) study, the findings are more representative of global populations. Such diversity enhances the reliability and applicability of genetic insights, paving the way for personalized medicine.
The findings also have implications for understanding age-related brain changes. Genetic variants associated with brain aging, such as those influencing tau pathology and oxidative resistance, could help identify individuals at risk for neurodegenerative diseases like Alzheimer’s. Early detection and intervention strategies could mitigate the progression of such conditions.
The implications of this research extend beyond academic curiosity. Identifying genetic variants associated with brain volumes can inform strategies for early diagnosis and treatment of neurological and psychiatric conditions. For example, understanding how certain genes influence brain aging could lead to interventions that mitigate the effects of neurodegenerative diseases.
“This paper, for the first time, pinpoints exactly where these genes act in the brain,” said Thompson. “This provides the beginnings of a roadmap for where to intervene.”
As scientists delve deeper into the genetic architecture of the brain, the potential to unlock treatments for conditions like ADHD, Parkinson’s, and other brain-related disorders becomes increasingly tangible. By bridging genetics, neuroimaging, and diverse global datasets, researchers are taking significant strides toward understanding the most complex organ in the human body.
Moreover, the study paves the way for developing targeted therapies. With a clearer understanding of genetic variants and their impact on brain regions, future treatments could address the root causes of structural abnormalities. These advancements hold promise not only for treating existing conditions but also for preventing their onset.
The integration of genetic data with clinical practices could revolutionize how brain-related diseases are managed. From personalized treatment plans to predictive risk assessments, the possibilities are vast. However, realizing this potential will require continued investment in large-scale collaborative research and the development of advanced analytical tools.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Groundbreaking brain study links Parkinson’s disease and ADHD appeared first on The Brighter Side of News.