Endocannabinoids, molecules naturally produced in the brain, play a critical role in regulating appetite, energy use, and physical activity. They interact with specific receptors, such as CB1, to influence behaviors related to food and movement.
Scientists have long known that manipulating this system can affect weight gain. However, targeting it safely has remained a challenge since the withdrawal of the anti-obesity drug Rimonabant over serious side effects like depression and anxiety.
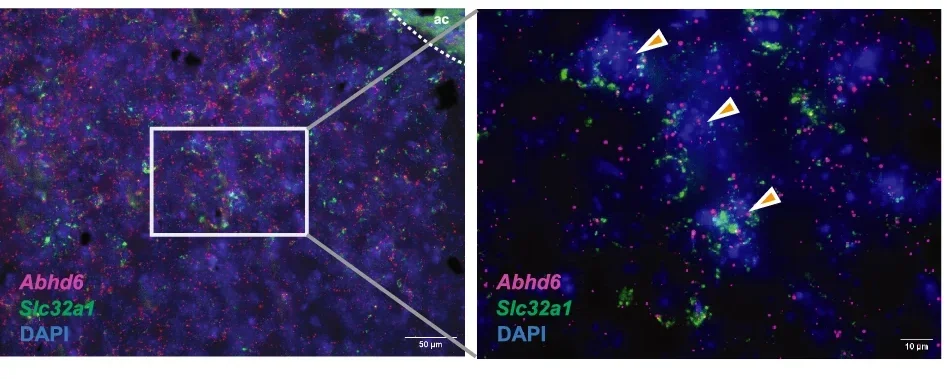
New research sheds light on a promising path. A key enzyme called ABHD6, which breaks down a specific endocannabinoid known as 2-arachidonoylglycerol (2-AG), may hold the answer to safely regulating weight and metabolism.
Studies in mice show that inhibiting this enzyme in certain brain regions protects against obesity, reduces sedentary behavior, and boosts physical activity. The findings offer potential for new therapies to treat obesity and related metabolic disorders.

Stephanie Fulton, a professor of medicine at Université de Montréal, and her team have been exploring how the brain’s endocannabinoid system affects appetite and activity levels. Their most recent study, published in Nature Communications, reveals that ABHD6 plays a key role in two areas of the brain—the nucleus accumbens and the ventral tegmental area—both critical for reward processing and motivation.
“We expected that increasing 2-AG levels would stimulate food intake by increasing cannabinoid signaling,” Fulton explained. “Paradoxically, we found that when we deleted the gene encoding ABHD6 in the nucleus accumbens, there was less motivation for food and greater interest in physical activity.”
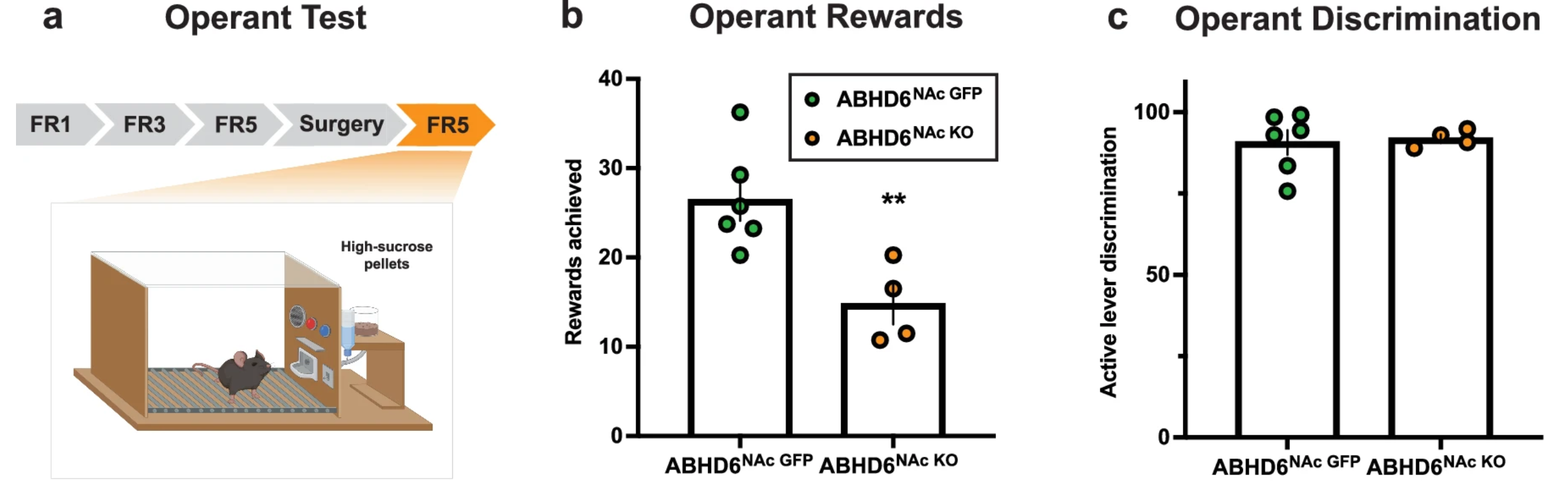
In the study, mice without ABHD6 in the nucleus accumbens chose exercise over food. When given access to running wheels, these mice spent significantly more time running compared to a control group. The control mice, by contrast, gained weight and became lethargic on a high-fat diet. Blocking ABHD6 not only increased activity but also protected the mice from diet-induced obesity.
The nucleus accumbens is a region of the brain known for its role in processing reward, including the pleasurable effects of food. Normally, endocannabinoids like 2-AG enhance motivation to eat by activating CB1 receptors in this area. ABHD6, located near the site where 2-AG is produced, regulates its levels.
Related Stories
By breaking down 2-AG, ABHD6 limits CB1 signaling. Without this enzyme, 2-AG accumulates, altering the reward system in surprising ways—mice showed less interest in food and a greater drive to be active.
However, the effects of ABHD6 inhibition depend on the specific brain region targeted. The ventral tegmental area (VTA), which sends dopamine signals to the nucleus accumbens, also plays a role in feeding and motivation.
In the VTA, deleting ABHD6 in dopamine neurons produced a different effect. Mice became more motivated to seek food, but only under certain conditions—specifically when their energy reserves were low. When not hungry, the same manipulation reduced their overall activity levels.
These findings highlight the complexity of the endocannabinoid system and its regulation of behavior. Endocannabinoid signaling in the VTA and nucleus accumbens controls dopamine release, which influences both reward-seeking and movement.
2-AG, in particular, targets CB1 receptors located on inhibitory and excitatory nerve terminals in these regions. By reducing inhibition of dopamine neurons, CB1 activation increases dopamine release, reinforcing reward-related behaviors such as eating.
“The ability to target specific neuronal pathways in the brain to control weight is crucial,” Fulton said. “Depending on the area of the brain, inhibiting ABHD6 can have opposite effects.”
This specificity is key to developing safe and effective therapies. Earlier efforts to target the endocannabinoid system, such as Rimonabant, lacked this precision. While Rimonabant successfully reduced appetite and promoted weight loss, it also affected mood, leading to depression and suicidal thoughts in some patients. Unlike CB1 receptor blockers, ABHD6 inhibitors appear to act in a more targeted manner without disrupting emotional behavior.
“In our study, we show that mice with ABHD6 inhibition did not exhibit signs of anxiety or depressive behavior,” Fulton noted. “That’s an important step toward identifying safer treatments.”
The team’s findings build on earlier work that suggested ABHD6 inhibition has broad metabolic benefits. In 2016, research led by Marc Prentki at Université de Montréal showed that blocking ABHD6 throughout the body reduced weight gain and protected against diabetes. This earlier discovery raised questions about ABHD6’s role in the brain, where it affects appetite and physical activity.
The latest study goes further by pinpointing how ABHD6 influences behavior in specific brain regions. By using viral gene knockout techniques in mice, researchers were able to delete ABHD6 in targeted neurons while avoiding unintended effects on development. The results reveal distinct roles for ABHD6 in the nucleus accumbens and VTA, showing how it shapes both food motivation and physical activity in response to energy states.
For instance, deleting ABHD6 in nucleus accumbens neurons led to weight loss and increased exercise, even on a high-fat diet. In contrast, removing ABHD6 from VTA neurons decreased activity but heightened food-seeking behavior when energy was low. These region-specific effects underscore the complexity of the brain’s endocannabinoid system and the importance of targeting it with precision.
While the study provides promising insights, more work is needed to determine whether these mechanisms operate the same way in humans. Fulton’s team is optimistic that ABHD6 inhibitors could offer a safer alternative for managing obesity and related metabolic disorders.
“Our work paves the way for therapies that could help fight obesity without the severe side effects seen with earlier drugs,” Fulton said.
Efforts are already underway to screen ABHD6 inhibitors for potential therapeutic use. If these molecules prove effective in humans, they could provide a much-needed tool for tackling the global obesity epidemic. Obesity is a major risk factor for type 2 diabetes, heart disease, and other metabolic conditions, affecting millions worldwide.
By unraveling the role of ABHD6 in the brain, researchers are bringing science one step closer to solving this challenge. The findings demonstrate how manipulating the endocannabinoid system can shift the balance between food intake and physical activity, offering new hope for those struggling with weight management.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Groundbreaking discovery targets key brain enzyme to treat obesity appeared first on The Brighter Side of News.