Breast cancer remains a leading health crisis worldwide, claiming the lives of approximately 685,000 women in 2020 alone. This complex disease is not a single entity but encompasses multiple subtypes, each defined by unique molecular traits.
The most prevalent form, estrogen receptor-positive (ER+) breast cancer, constitutes nearly 80% of cases. However, despite advancements in treatment, almost half of ER+ breast cancer patients experience a relapse, underscoring the urgency for innovative therapeutic strategies.
Breast cancer subtypes are primarily categorized by the presence or absence of three key receptors: estrogen receptor (ER), progesterone receptor (PR), and the epidermal growth factor receptor 2 (HER2). These molecular characteristics determine the behavior of the cancer and its response to treatments.
Endocrine therapies, which target ER signaling, are the standard approach for ER+ cancers. Yet, resistance to these therapies emerges in nearly half of the patients, complicating treatment and survival outcomes.
Recent research has shed light on the role of G-protein coupled receptors (GPCRs) in cancer progression. GPCRs are a vast family of cell surface receptors involved in critical physiological processes, from immune responses to metabolism.
Despite their prominence as drug targets in other fields, their application in cancer treatment has lagged. Emerging evidence highlights the significance of certain GPCRs, such as CXCR4 and LGR5, in tumor biology, pointing to new opportunities for therapeutic intervention.
Among the GPCR-associated proteins, the G12 subfamily, particularly Gα12 and Gα13, has drawn attention for its role in cancer dynamics.
Related Stories
Historically, Gα13 was considered a promoter of tumor growth, primarily in aggressive subtypes like triple-negative breast cancer (TNBC). However, groundbreaking research from Duke-NUS Medical School challenges this narrative. In ER+ breast cancer, Gα13 appears to function as a tumor suppressor, a discovery that overturns long-held assumptions.
Dr. Lalitha Subramanyan, a lead researcher on the study, explained: “Our findings challenge the previous notion that Gα13 universally promotes cancer growth across different tumor types. Instead, we found evidence suggesting that Gα13 may help disrupt harmful pathways in ER+ breast cancer, potentially slowing or stopping the growth of cancer cells.”
This study, published in Breast Cancer Research, marks the first identification of Gα13’s protective role in solid tumors.
Gα13 operates as a molecular messenger, transmitting signals from the cell surface to its interior, influencing growth and survival pathways.
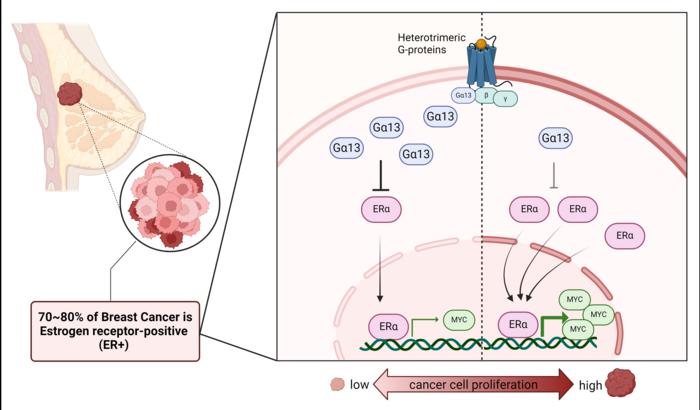
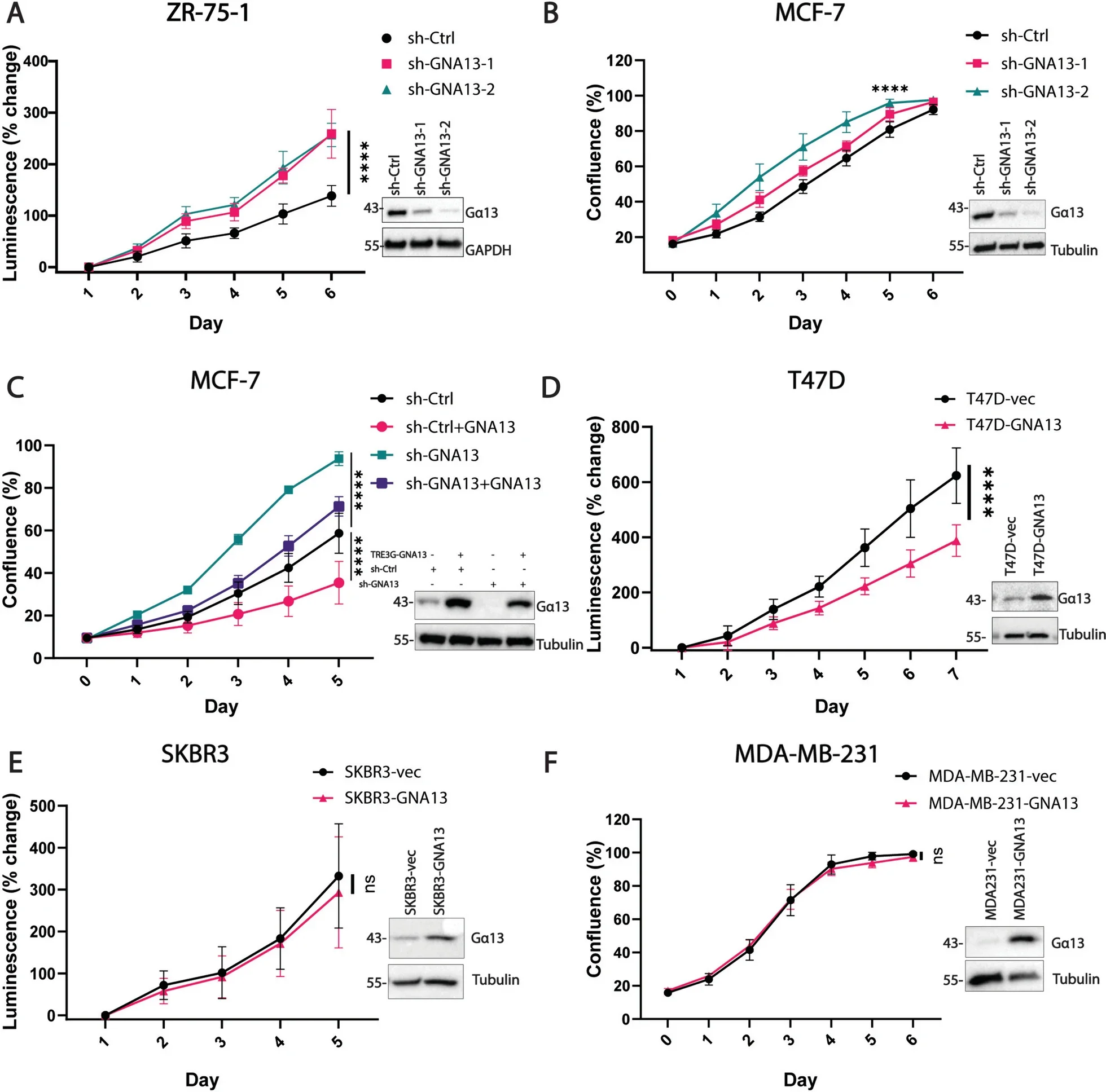
In ER+ breast cancer, researchers discovered that Gα13 negatively regulates the MYC oncogene, a critical driver of cancer cell proliferation. This regulation depends on estrogen signaling, revealing a novel intersection between Gα13 and hormonal pathways.
Upregulation of MYC is a well-documented mechanism behind endocrine therapy resistance in ER+ cancers. By controlling MYC levels, Gα13 offers a potential avenue to counteract therapy-resistant cases.
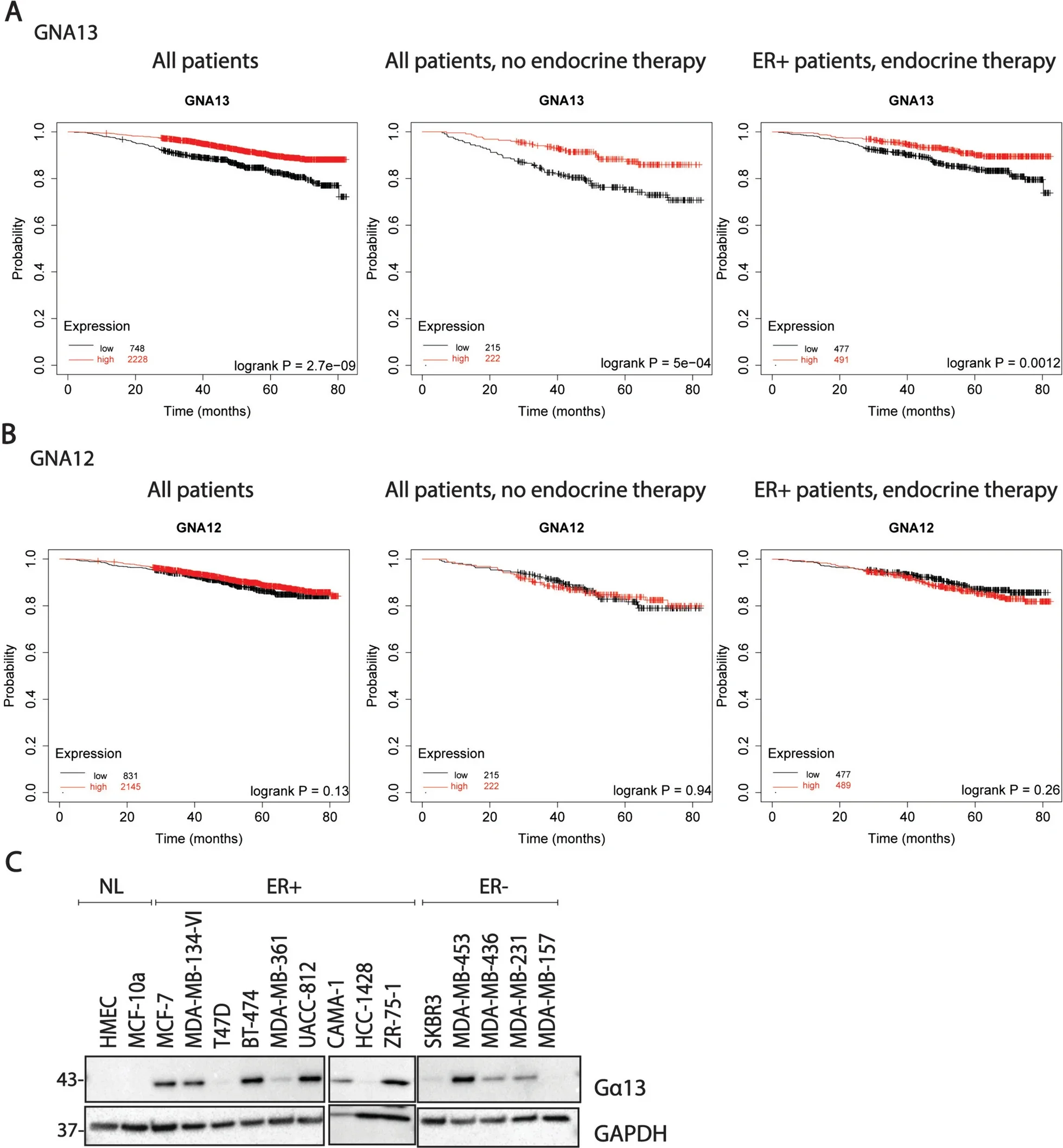
The study also revealed a correlation between lower Gα13 expression and poorer survival outcomes in ER+ breast cancer patients, further emphasizing its protective role.
Professor Mei Wang, a co-author of the study, highlighted the implications of this discovery: “While treatments for ER+ breast cancers primarily target ER signaling, nearly half of these patients develop resistance to such therapy over time. The discovery of Gα13’s control over estrogen signaling and MYC function offers new ways to counter resistant ER+ breast cancers.”
The findings pave the way for more personalized approaches to breast cancer treatment. By examining Gα13 levels and related molecular markers, clinicians could better predict patient responses to therapy and tailor interventions accordingly. This personalized strategy could enhance treatment efficacy and improve survival rates for those battling ER+ breast cancer.
Associate Professor Yap Yoon Sim from the National Cancer Centre Singapore, who was not involved in the research, remarked: “These findings highlight the complexity of cancer biology and the need to understand the role of different molecules and pathways in various settings. It is hoped that this knowledge can facilitate the development of novel strategies to treat breast cancer in the near future.”
The Duke-NUS research team plans to extend their investigation into other hormone-sensitive cancers, exploring whether Gα13’s tumor-suppressive properties apply to additional cancer types. This broader perspective could unlock new therapeutic targets beyond breast cancer.
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, underscored the significance of this breakthrough: “Understanding these molecular mechanisms paves the way for targeted drug development, which could enhance the efficacy of breast cancer treatments and ultimately improve survival rates and quality of life for those affected by this devastating disease.”
As researchers continue to unravel the complexities of cancer biology, the discovery of Gα13’s unexpected role exemplifies the potential for innovative science to redefine treatment paradigms.
By integrating molecular insights with clinical strategies, the future of breast cancer therapy looks increasingly promising.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Lifechanging discovery reduces breast cancer recurrence appeared first on The Brighter Side of News.