The brain runs on energy. Every thought, memory, or emotion sparks countless signals between nerve cells. That communication demands a steady, powerful fuel supply. Without it, the system breaks down.
In Alzheimer’s, this fuel line begins to fail. The energy that neurons rely on to talk to one another weakens. As the power dwindles, memory fades and connections start to unravel.
A recent study, published in Advanced Science by researchers at Scripps Research, reveals what might be sabotaging this energy pipeline. Scientists looked deep into the machinery inside cells—the mitochondria. These tiny engines keep neurons alive and active. When they malfunction, the whole network suffers.
Using nerve cells grown from Alzheimer’s patient stem cells, researchers repaired this cellular damage. They zeroed in on faulty mitochondria. After intervention, many broken connections between neurons began to recover.
The decline in mitochondrial health has been tied to neurodegenerative diseases for years. In Alzheimer’s disease, that connection is especially strong. When these engines sputter, the mind starts to slip.
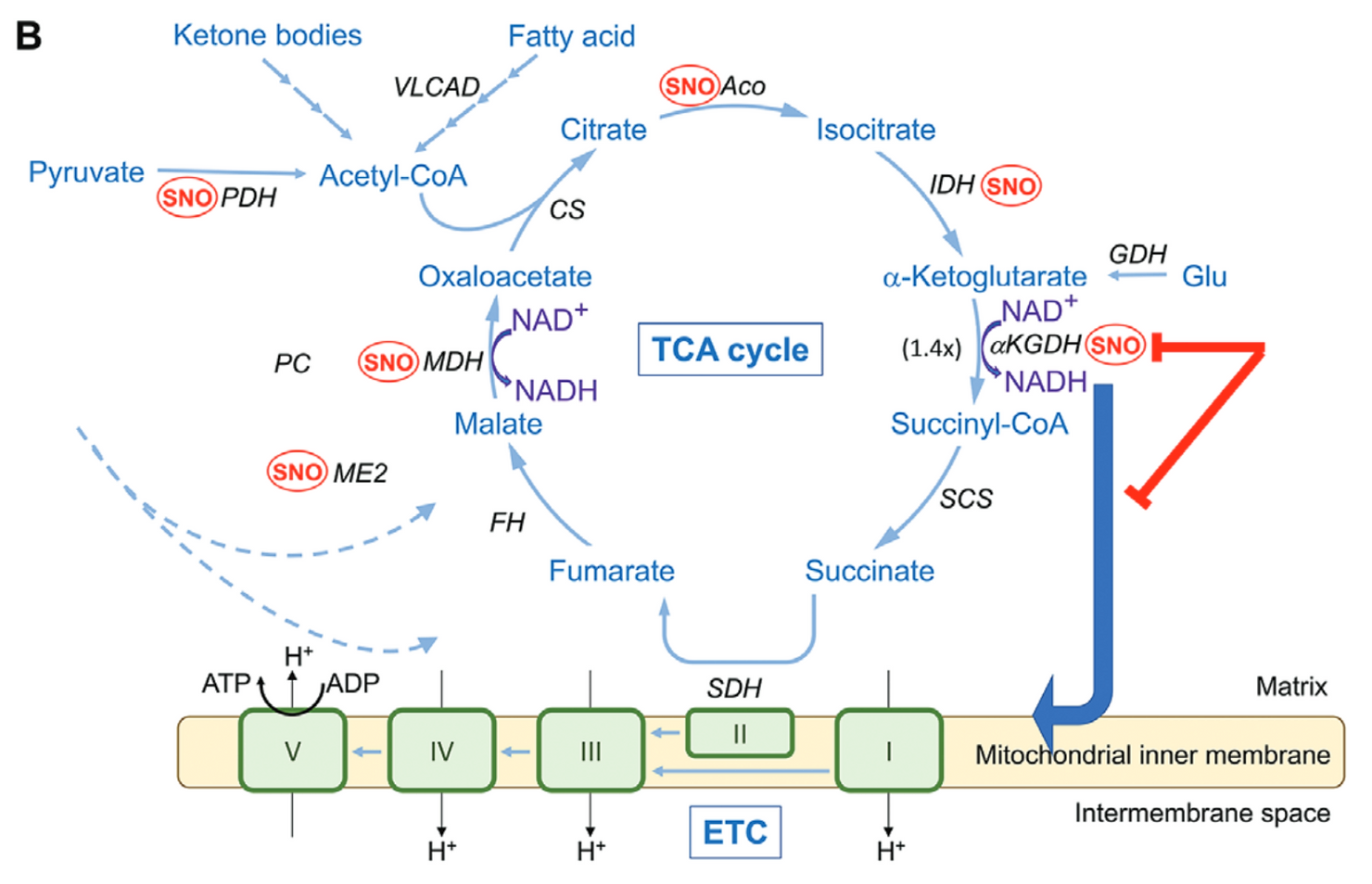
The root of the problem traces back to a breakdown in the Krebs cycle. Also known as the TCA cycle, it’s the process cells use to generate energy. Without it, neurons can’t function properly.
At the center of this breakdown is a rogue chemical change. It’s called S-nitrosylation. In this reaction, nitrogen and oxygen attach to sulfur on key proteins, disrupting how they work.
One enzyme hit hard by this change is α-ketoglutarate dehydrogenase, or αKGDH. It plays a major role in fueling cells. When it’s altered, the energy factory stalls.
In brains affected by Alzheimer’s, researchers found too many of these altered enzymes. They called the surge a “SNO-Storm”—a flood of damaged proteins that shuts down energy production at the source.
Related Stories
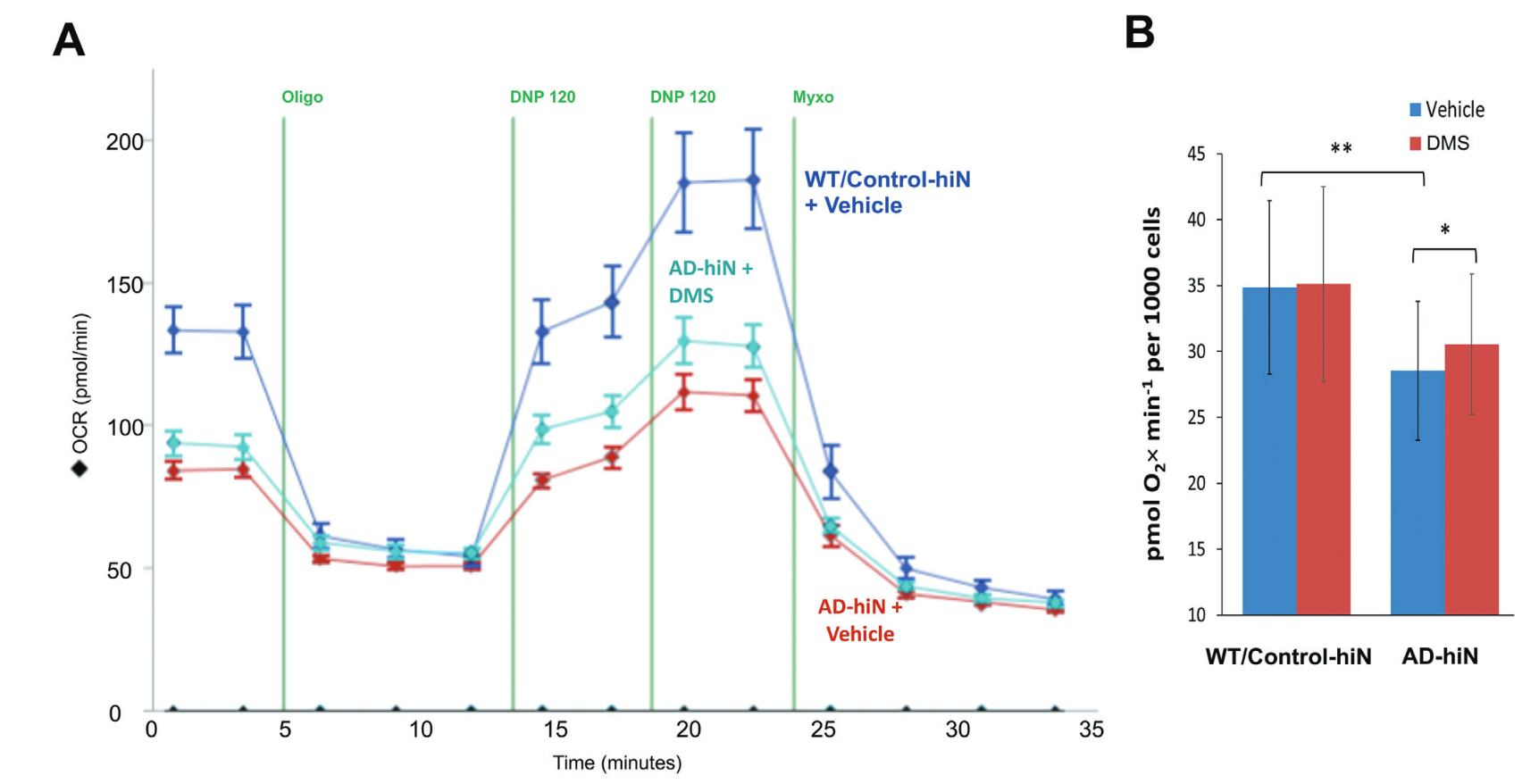
Using advanced techniques, such as 13C dynamic labeling and the Seahorse platform to measure oxygen consumption rates (OCR), the researchers pinpointed significant deficits in the TCA cycle.
These findings were confirmed in both human postmortem Alzheimer’s brains and in neurons derived from Alzheimer’s patient stem cells. The neurons exhibited reduced mitochondrial respiration and impaired energy production, correlating with the extensive S-nitrosylation of critical enzymes.
One key discovery was a bottleneck in the Krebs cycle, specifically in the production of succinate, a molecule crucial for generating ATP—the primary energy source of cells. This bottleneck prevents the mitochondria from producing adequate energy, compromising the survival of neurons and their intricate network of synapses.
The research team hypothesized that replenishing succinate could restore energy production. However, succinate faces challenges in crossing nerve cell membranes. To overcome this, the scientists used a succinate analog capable of penetrating cells effectively. This approach successfully repaired up to 75% of the lost synapses in their models, providing a potential pathway to rescue neuronal connectivity.
“Succinate is not a compound that people can now take as a treatment, but it’s proof-of-principle that you can re-energize the Krebs cycle,” explained Dr. Stuart Lipton, senior author of the study. He emphasized the need for further research to develop a safe and effective energy-restoring drug for humans.
The implications of this research extend far beyond the laboratory. Dr. Lipton, a seasoned clinical neurologist and the Step Family Foundation Endowed Professor at Scripps Research, has a track record of developing treatments for Alzheimer’s, including Namenda®. His team’s findings highlight mitochondrial metabolism as a promising therapeutic target for Alzheimer’s and related neurodegenerative diseases.
The study also underscores the importance of understanding the broader mechanisms underlying energy deficits in Alzheimer’s. Posttranslational modifications, such as S-nitrosylation, represent critical control points in bioenergetics. While this research focused on αKGDH, other enzymes within the TCA cycle may also be affected, necessitating further investigation.
Beyond their immediate findings, the researchers’ use of human-induced pluripotent stem cell (hiPSC) models provides a valuable tool for studying Alzheimer’s at the cellular level. By generating neurons from stem cells derived from Alzheimer’s patients, the team was able to replicate disease-relevant metabolic changes and identify therapeutic targets with high precision.
While the findings offer hope, the journey toward an effective treatment remains challenging. Developing drugs capable of safely restoring mitochondrial function will require rigorous testing and clinical trials. Additionally, the complexity of Alzheimer’s disease means that targeting energy deficits may need to be combined with other therapeutic strategies to address the multifaceted nature of the condition.
Dr. Lipton’s team is committed to advancing this line of research. By exploring innovative approaches to re-energize the Krebs cycle, they aim to halt disease progression and improve cognitive outcomes for patients. “We thought that if we could repair metabolic activity in the mitochondria, maybe we could salvage the energy production,” Dr. Lipton remarked. This vision reflects the determination of researchers worldwide to confront Alzheimer’s with scientific ingenuity and compassion.
In a world where Alzheimer’s disease robs millions of their memories and independence, breakthroughs like this bring a glimmer of hope.
By addressing the fundamental energy crisis in the brain, scientists are paving the way for treatments that could preserve not only neurons but also the connections that define our humanity.
Scientists do not yet fully understand what causes Alzheimer’s disease. There likely is not a single cause but rather several factors that can affect each person differently.
In 2010, the costs of treating Alzheimer’s disease were projected to fall between $159 and $215 billion. By 2040, these costs are projected to jump to between $379 and more than $500 billion annually.
Death rates for Alzheimer’s disease are increasing, unlike heart disease and cancer death rates that are on the decline.
Dementia, including Alzheimer’s disease, has been shown to be under-reported in death certificates and therefore the proportion of older people who die from Alzheimer’s may be considerably higher.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Lifechanging study restores brain function for Alzheimer’s patients appeared first on The Brighter Side of News.
Leave a comment
You must be logged in to post a comment.