Researchers from the University of Leicester, working with international collaborators, have unraveled a key mechanism by which dietary cholesterol is absorbed into cells. This discovery, published in Science, promises innovative therapeutic pathways to control cholesterol uptake, potentially reducing cardiovascular disease rates globally.
Cholesterol, a fatty substance in the bloodstream, is essential for various bodily functions. Synthesized by the liver and present in foods like red meat and dairy, cholesterol becomes harmful in excess. High levels can clog arteries, increasing the risk of heart disease and stroke. Controlling cholesterol absorption is thus crucial for maintaining heart health.
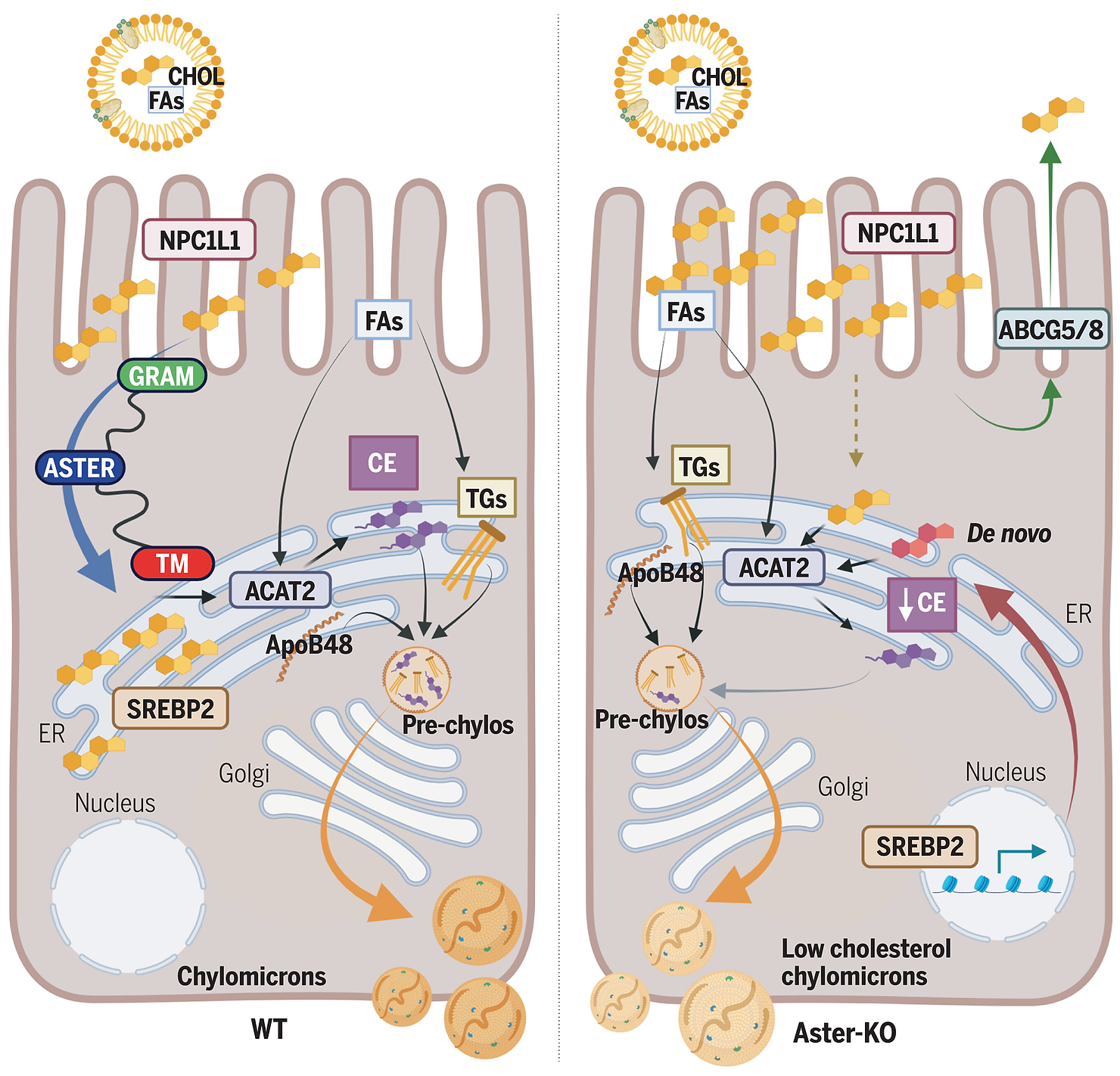
The small intestine plays a vital role in systemic cholesterol regulation, absorbing dietary cholesterol and packaging it into molecules called chylomicrons for transport in the bloodstream.
A key player in this process is a protein, Niemann-Pick C1 Like 1 (NPC1L1). This protein transfers cholesterol from the gut lumen to the plasma membrane of intestinal cells, initiating absorption. However, until recently, how cholesterol moves from this initial point to the endoplasmic reticulum (ER)—where it undergoes chemical modification—was unknown.
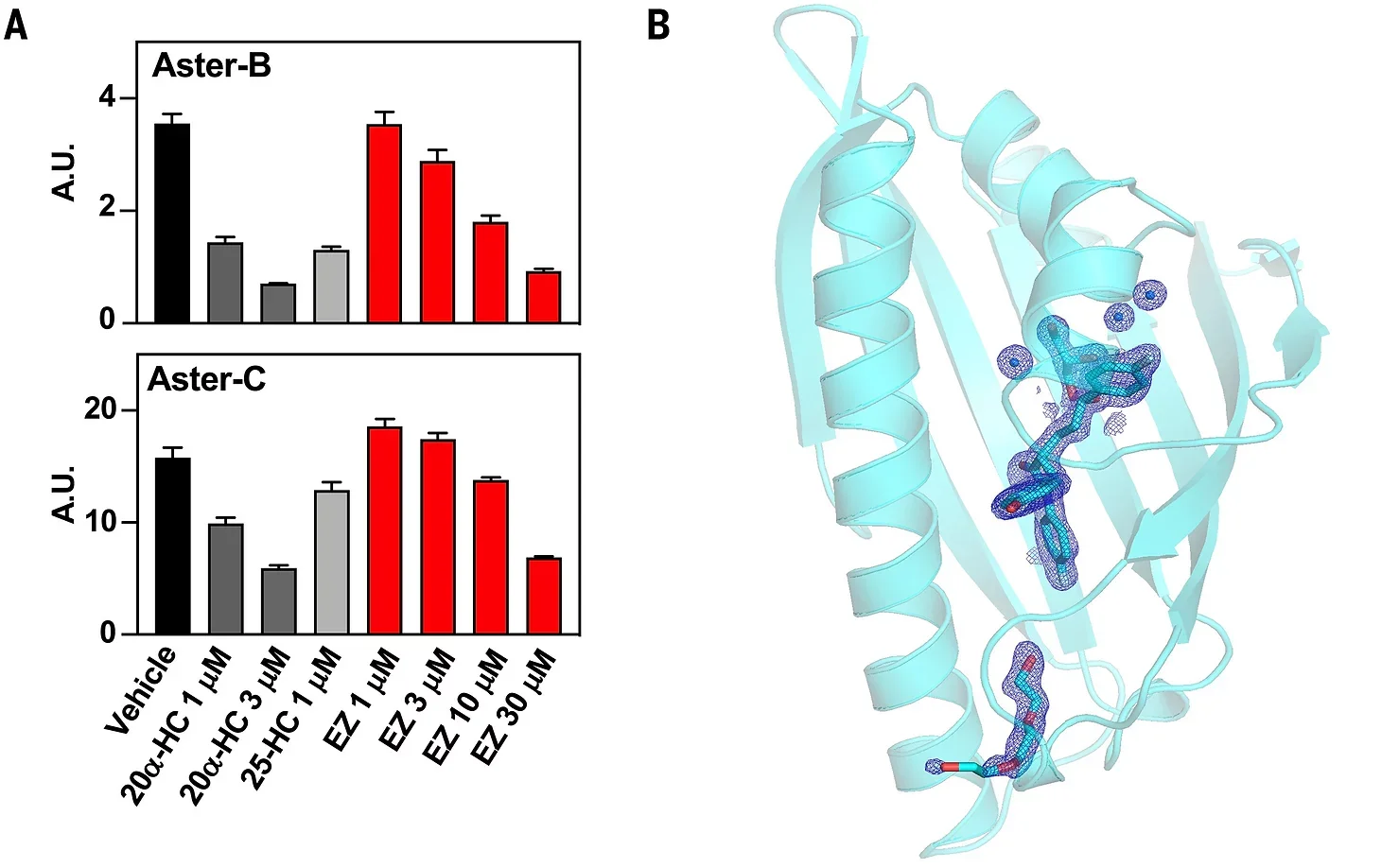
The team identified Aster proteins, specifically Aster-B and Aster-C, as crucial for the next steps in cholesterol transport. These proteins shuttle cholesterol from the cell membrane to the ER, enabling its conversion into cholesterol ester for further distribution.
Mice lacking Asters showed reduced cholesterol absorption and were protected from diet-induced high cholesterol. These findings establish Asters as key targets for developing cholesterol-lowering drugs.
This research highlights the potential for targeting the NPC1L1-Aster pathway to control cholesterol absorption. A well-known cholesterol-lowering drug, Ezetimibe, blocks NPC1L1 and reduces cholesterol uptake, validating the small intestine as a viable therapeutic target.
Related Stories
Ezetimibe binds to NPC1L1, preventing cholesterol from entering the cell membrane. While effective, it doesn’t address the role of Asters. By inhibiting Aster proteins, researchers propose a complementary method to further reduce cholesterol absorption.
Professor John Schwabe, Director of the Institute for Structural and Chemical Biology at the University of Leicester, emphasized the importance of these findings. “This breakthrough is the result of a long-lasting collaboration and forms part of an international effort to combat cardiovascular disease and stroke. Understanding how cholesterol moves within cells and tissues is essential for designing targeted drugs to address these pressing health issues.”
This research was funded by the Leducq Foundation, which granted $6 million to a global network of laboratories focused on cholesterol transport. The project exemplifies how international collaborations can tackle complex health challenges. Researchers examined cholesterol absorption and metabolism from multiple angles, providing a comprehensive understanding of the processes involved.
Dr. Beatriz Romartinez-Alonso, a postdoctoral researcher, shared her enthusiasm for the project, saying, “This has been a great project to work on—discovering new science highly relevant to human health.”
The broader implications of these findings extend beyond the laboratory. Cardiovascular disease remains a leading cause of death worldwide.
High cholesterol levels are a modifiable risk factor, and the ability to control dietary cholesterol absorption could revolutionize prevention strategies. By targeting Aster proteins, scientists aim to develop drugs that reduce cholesterol uptake more effectively than current treatments.
The discoveries mark a turning point in the fight against heart disease. By understanding the interplay between NPC1L1 and Aster proteins, researchers are opening new avenues for cholesterol management. Reducing cholesterol absorption not only lowers the risk of heart attacks but also addresses other cholesterol-related diseases.
Professor Schwabe added, “If we can prevent some cholesterol from being absorbed into our cells, we may ultimately prevent high cholesterol and reduce risks of heart attacks and strokes. This research has the potential to save lives.”
Ezetimibe’s success highlights the importance of targeting cholesterol transport proteins. With further advancements, drugs designed to inhibit Aster proteins could work alongside existing therapies, providing a comprehensive solution to cholesterol management.
This breakthrough underscores the importance of continued research into cholesterol transport mechanisms. By investing in understanding these pathways, scientists are paving the way for a future where heart disease is less prevalent, and lives are saved.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Lifesaving cholesterol discovery could prevent heart disease and stroke appeared first on The Brighter Side of News.