Electric vehicles are redefining transportation, but battery lifespan remains a key hurdle. Lithium-ion batteries, the backbone of most EVs, degrade over time. This leads to costly replacements and growing environmental concerns. But a new breakthrough in battery design might pave the way for batteries that last up to one million kilometers without needing to be swapped out.
The heart of the problem lies in how these batteries charge and discharge. Inside, nickel-based cathode materials store lithium ions. These materials are made of many tiny crystals. Over time, repeated charging and discharging causes the crystals to break apart. This weakens the structure and reduces the battery’s ability to hold a charge.
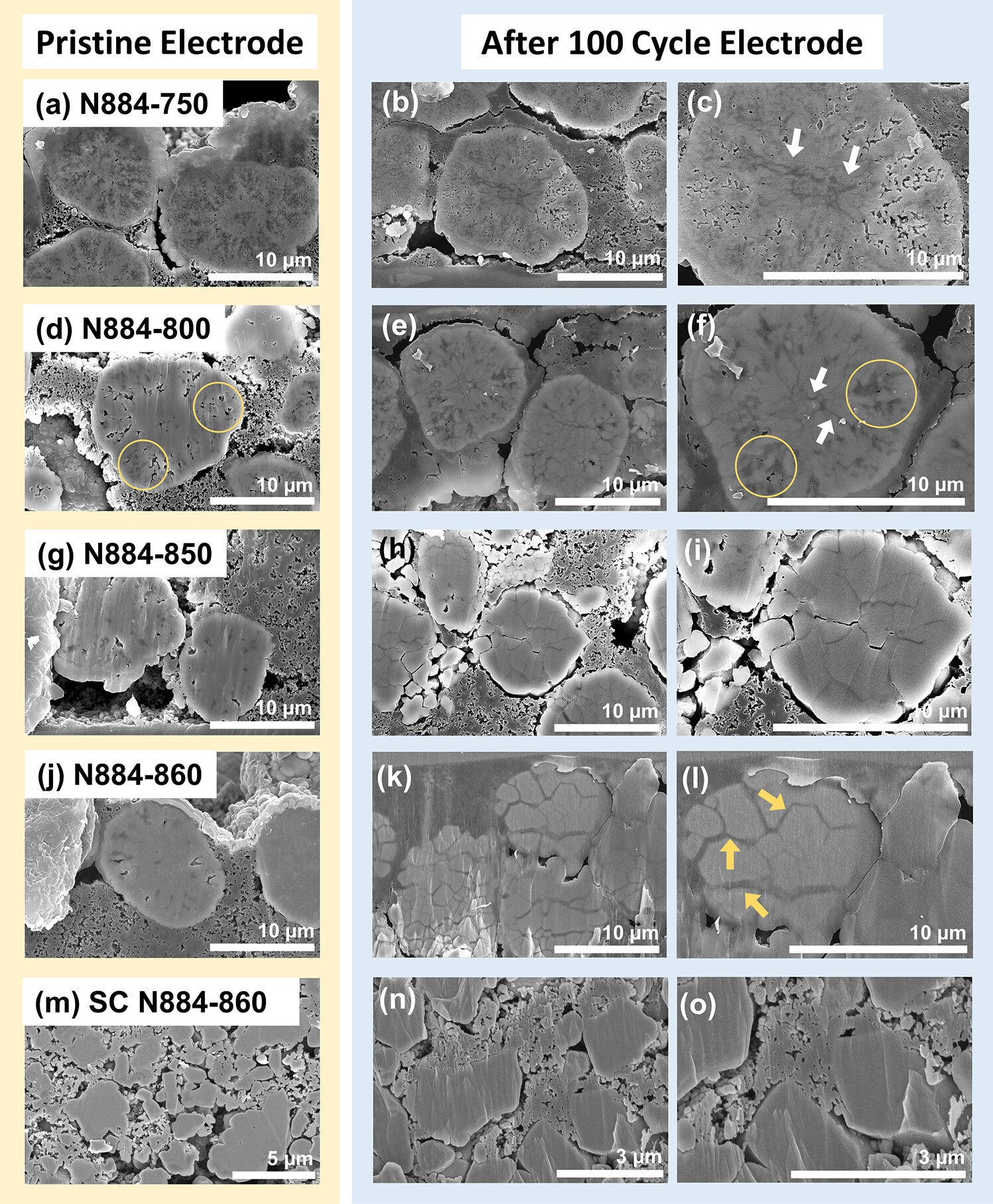
Researchers have been looking for ways to extend battery life. One promising solution involves reshaping how these nickel cathodes are made. Instead of using many small crystals, scientists explored forming the material as a single large crystal. Unlike their multi-crystal counterparts, single-crystal materials are more resistant to damage, potentially offering a longer-lasting solution for EV batteries.
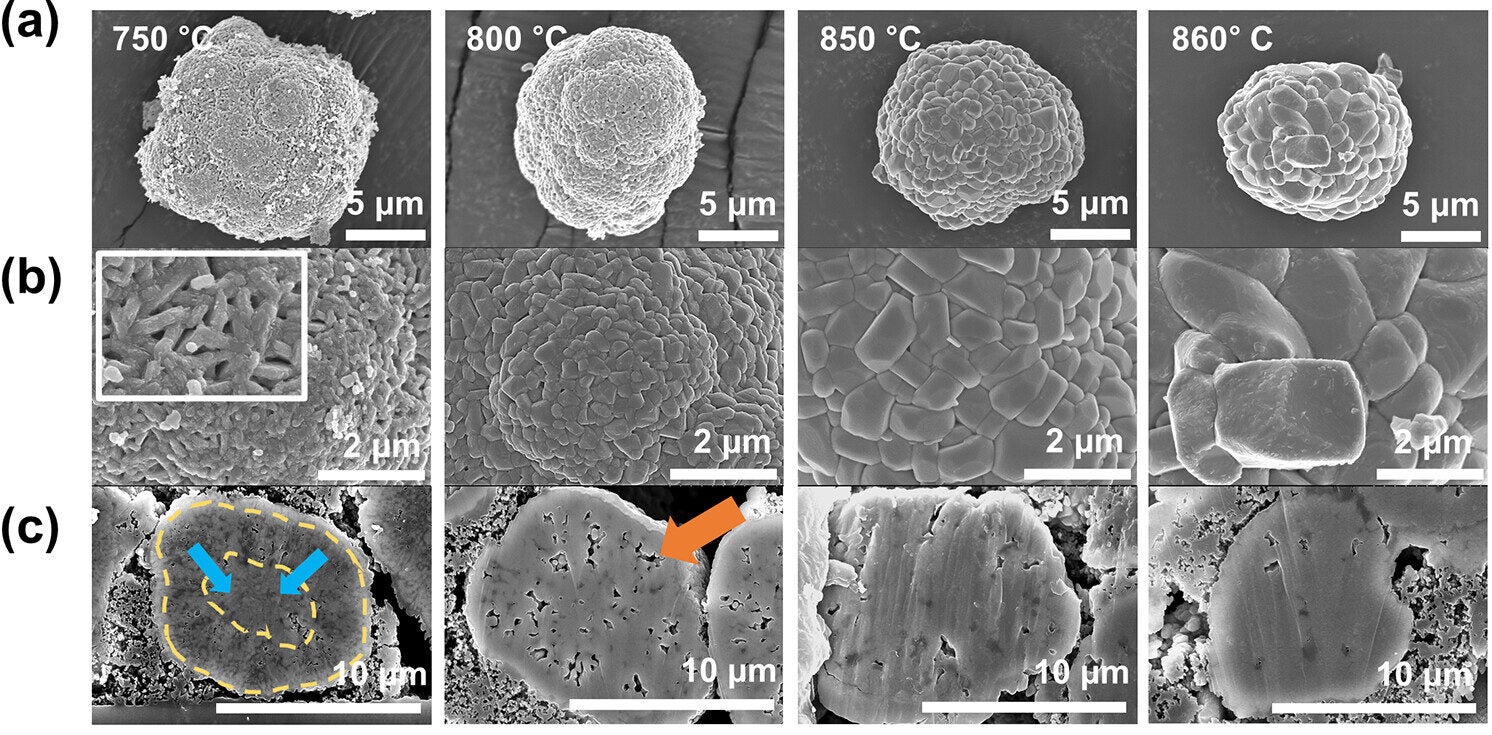
To understand what makes these crystals more durable, researchers at Pohang University of Science & Technology in South Korea studied how temperature affects their structure. Led by Kyu-Young Park, the team tested various heat levels to identify the point where high-quality single crystals could form.
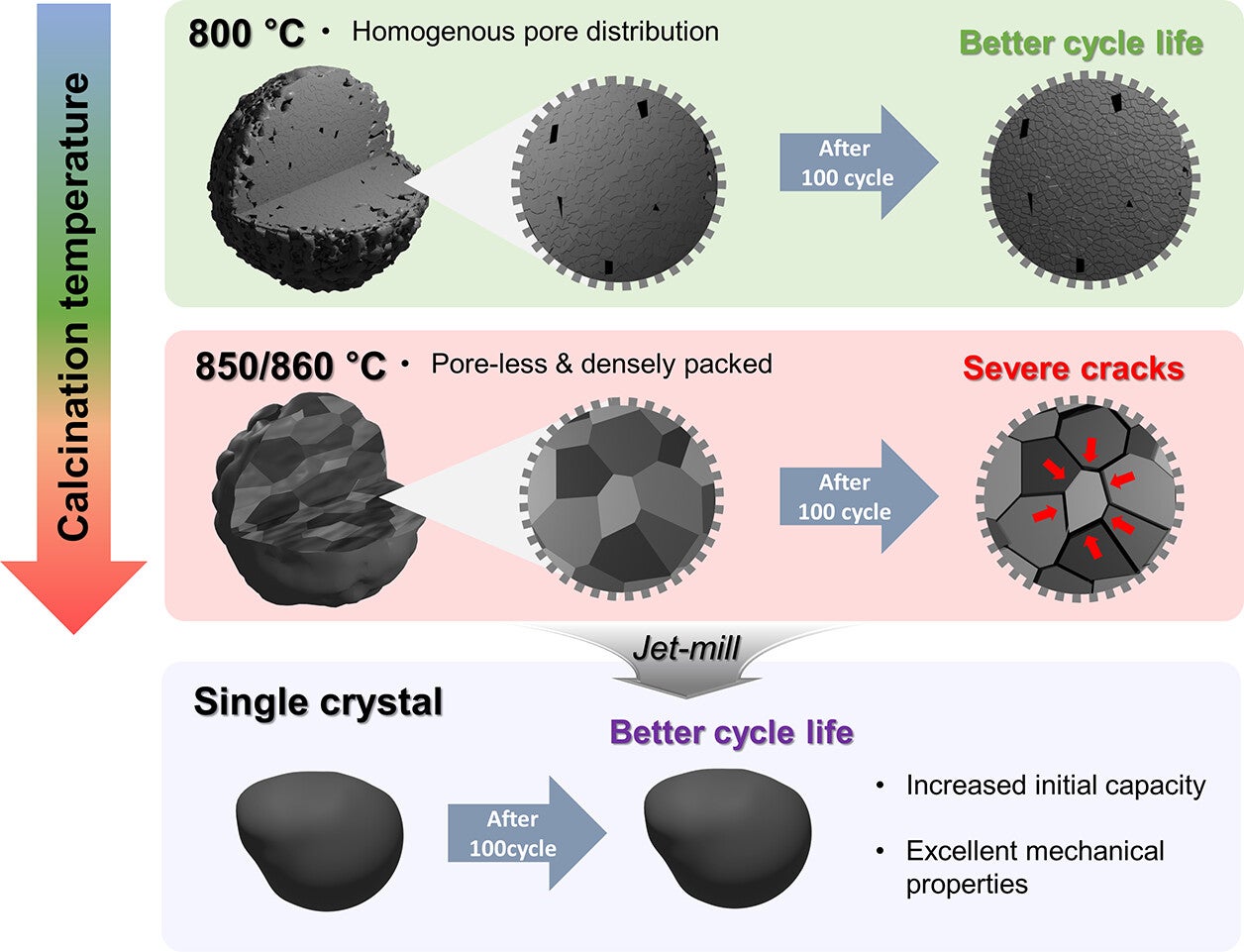
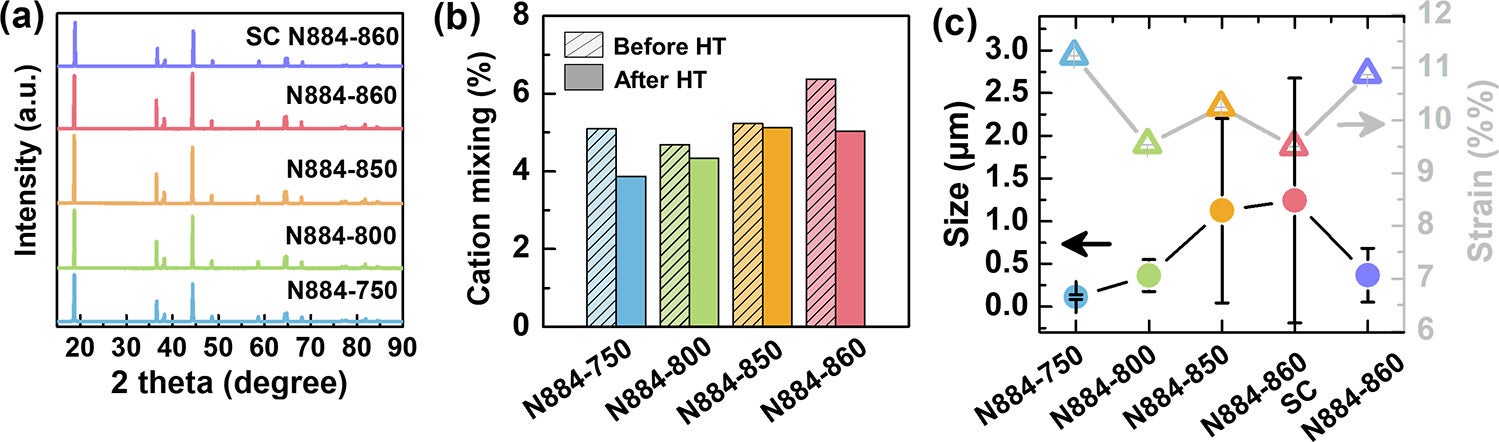
They found that at temperatures above 850 °C, the internal structure of the cathode undergoes a major shift. Tiny grains within the material fuse and grow, while air pockets between them vanish. This process, known as densification, creates a hardened structure that resists wear and tear.
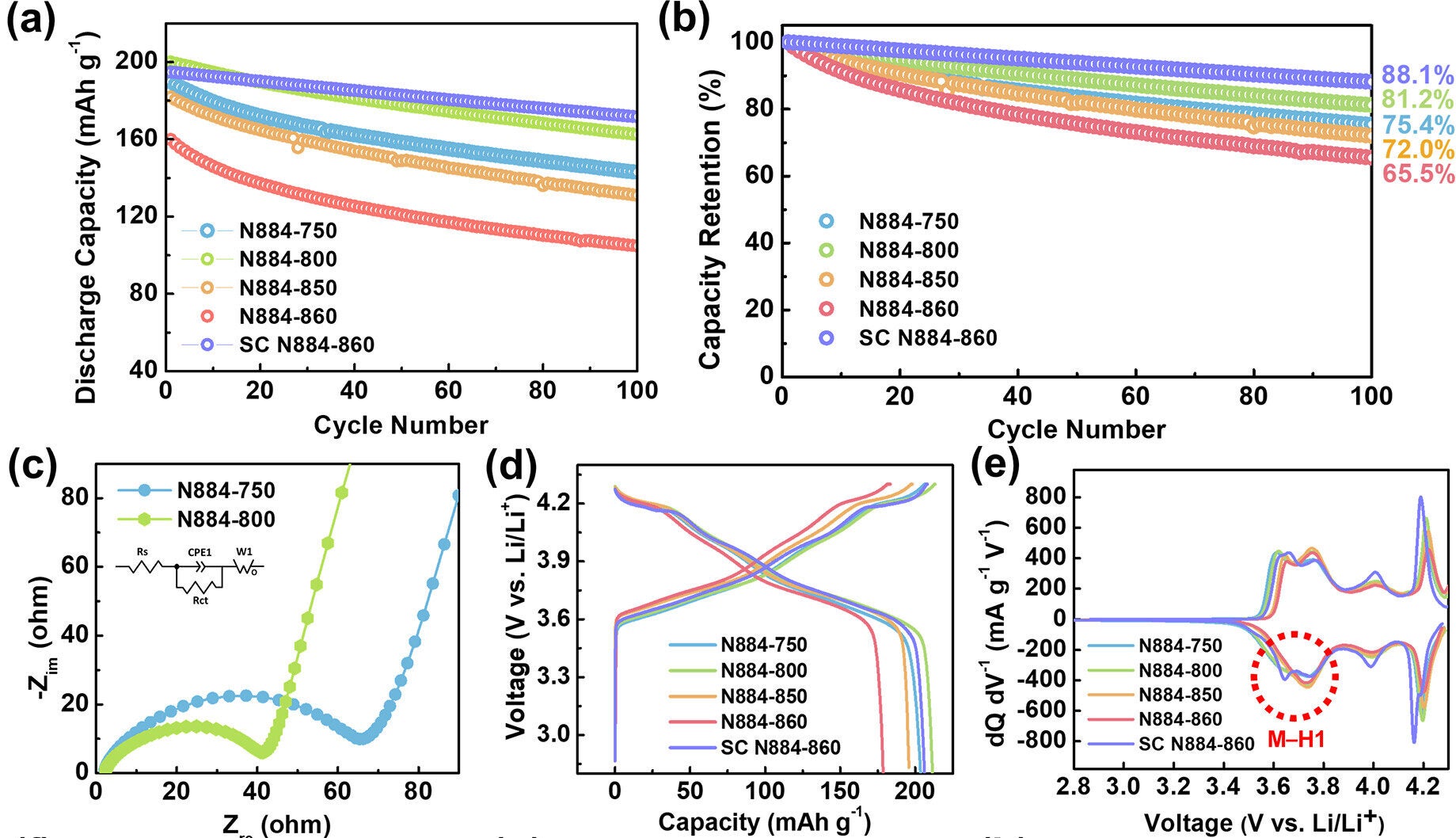
However, when polycrystalline materials are made at this high temperature, they become too dense. With fewer pores to absorb stress, they’re more likely to crack during use. In contrast, single-crystal cathodes formed at the same temperature hold up better, avoiding the fractures that plague their polycrystalline counterparts.
Related Stories
“Our new synthesis strategy enhances the durability of nickel-based cathode materials,” Park explained. “We will continue our research to make secondary batteries for electric vehicles cheaper, faster, and longer-lasting.”
This research, published in ACS Applied Materials & Interfaces, highlights the link between calcination temperature, microstructure, and long-term battery performance. The findings could transform how EV batteries are designed, improving not just durability but also affordability and sustainability.
Cathode materials are key to improving energy density, which directly affects driving range. One approach has been to increase the amount of nickel in the cathode. While this boosts energy capacity, it also introduces problems. High-nickel cathodes tend to develop cracks and suffer from faster performance decline over time.

These failures result from how lithium ions interact with the crystal structure. When too much lithium leaves the material during discharge, the structure collapses in one direction. This stress leads to tiny cracks between grains, weakening the material and reducing how many charge cycles the battery can handle.
To solve this, researchers have tried different strategies—adding coatings, doping the material with other elements, and designing better internal structures. But one of the most effective methods has turned out to be controlling the cathode’s microstructure. A well-designed structure with balanced grain size and pore distribution can absorb stress more evenly and slow down degradation.
That’s where calcination temperature comes into play. Below 850 °C, the material retains small, well-distributed pores that cushion stress. But once that threshold is crossed, the structure changes dramatically. Polycrystalline cathodes lose their pores and become brittle. On the other hand, single-crystal cathodes made at this same high temperature avoid cracking because they have no grain boundaries, offering much better durability.
This suggests that battery life hinges more on how the cathode is structured than on how perfect the crystal is. It also opens the door for longer-lasting EV batteries that don’t sacrifice power for endurance.
Even with better battery performance, another challenge looms—what happens when EV batteries reach the end of their life? As more vehicles hit the road, the number of used batteries is set to surge. If not handled properly, these batteries can cause serious environmental damage.
Lithium-ion batteries contain heavy metals like cobalt, nickel, and manganese. If these materials leak into the ground, they can poison soil and water. Mining new raw materials is also a major environmental burden, consuming large amounts of water and energy while leaving behind toxic waste.
Recycling these batteries is not easy. It’s expensive and energy-intensive, requiring specialized equipment. Still, scientists are working on better ways to recover valuable metals. One method, called hydrometallurgy, uses chemicals to dissolve and separate metals for reuse. Another method—direct recycling—restores parts of the battery, like the cathode, without breaking them down completely.
Many used EV batteries still hold 70–80% of their original storage power. These can be repurposed for less demanding uses, like storing energy from solar or wind farms. Researchers are exploring how to give these batteries a “second life,” reducing waste and extending their usefulness.
There’s also a push to develop new types of batteries that are easier to recycle. Solid-state and sodium-ion batteries are two promising options. They use safer materials and simpler designs, which could lower the cost and impact of battery disposal.
Governments are stepping in, too. Some are creating rules that require battery makers to take back used batteries. Others are offering incentives to encourage recycling and building better systems for collecting and processing old units.
The path to sustainable transportation doesn’t end with building better batteries—it also means managing their entire lifecycle. That includes how they’re made, used, and eventually discarded.
By understanding the role of microstructure in battery performance, researchers have taken a major step forward. Single-crystal cathodes produced at critical temperatures could offer longer-lasting and more reliable energy storage for electric vehicles. This would reduce the need for frequent replacements and ease pressure on global supply chains.
At the same time, improving recycling systems and repurposing spent batteries can help solve the growing waste problem. These efforts not only reduce environmental harm but also make electric vehicles more attractive and affordable in the long run.
With innovations in material design, recycling, and policy, the future of EVs looks more sustainable than ever. If batteries can reliably last a million kilometers, it will mark a turning point for clean energy and transportation around the world.
The disposal of electric vehicle (EV) car batteries poses significant environmental challenges. These batteries, typically made from lithium-ion materials, contain toxic metals like cobalt, nickel, and manganese, which can leak into the environment if improperly disposed of.
Additionally, the extraction and refining of raw materials for new batteries are resource-intensive and environmentally damaging. Here are the main issues and how scientists are addressing them:
Through these innovations, scientists aim to reduce the environmental impact of EV batteries and create a more sustainable lifecycle for electric vehicles.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post New EV battery tech could power cars for up to 1 million kilometers appeared first on The Brighter Side of News.