Plastics are everywhere, shaping industries with their durability, low cost, and lightweight properties. Over the last seven decades, plastic production has skyrocketed from just 2 million tons per year to a staggering 368 million tons. This number is expected to quadruple by 2050.
The United States alone generated 35.7 million tons of plastic waste in 2018, representing 12.2% of all municipal solid waste. Despite increasing production, recycling rates remain low. In the European Union, only 41% of plastic packaging waste was recycled in 2019. This gap leads to plastic accumulating in oceans, rivers, and landfills, causing long-term environmental damage.
Polyethylene terephthalate (PET) is one of the most widely used plastics, essential in beverage bottles, food packaging, and textiles. With global production reaching nearly 70 million tons per year, PET dominates the beverage industry, holding 67% of the market. However, excessive use and poor recycling rates mean PET makes up 12% of solid waste.
The material’s reliance on fossil fuels for production further intensifies environmental concerns. Recycling PET efficiently could significantly reduce landfill waste, cut down on carbon emissions, and save massive amounts of energy. A single ton of recycled PET can save 7,200 kWh of energy and prevent 5.1 tons of carbon dioxide emissions.
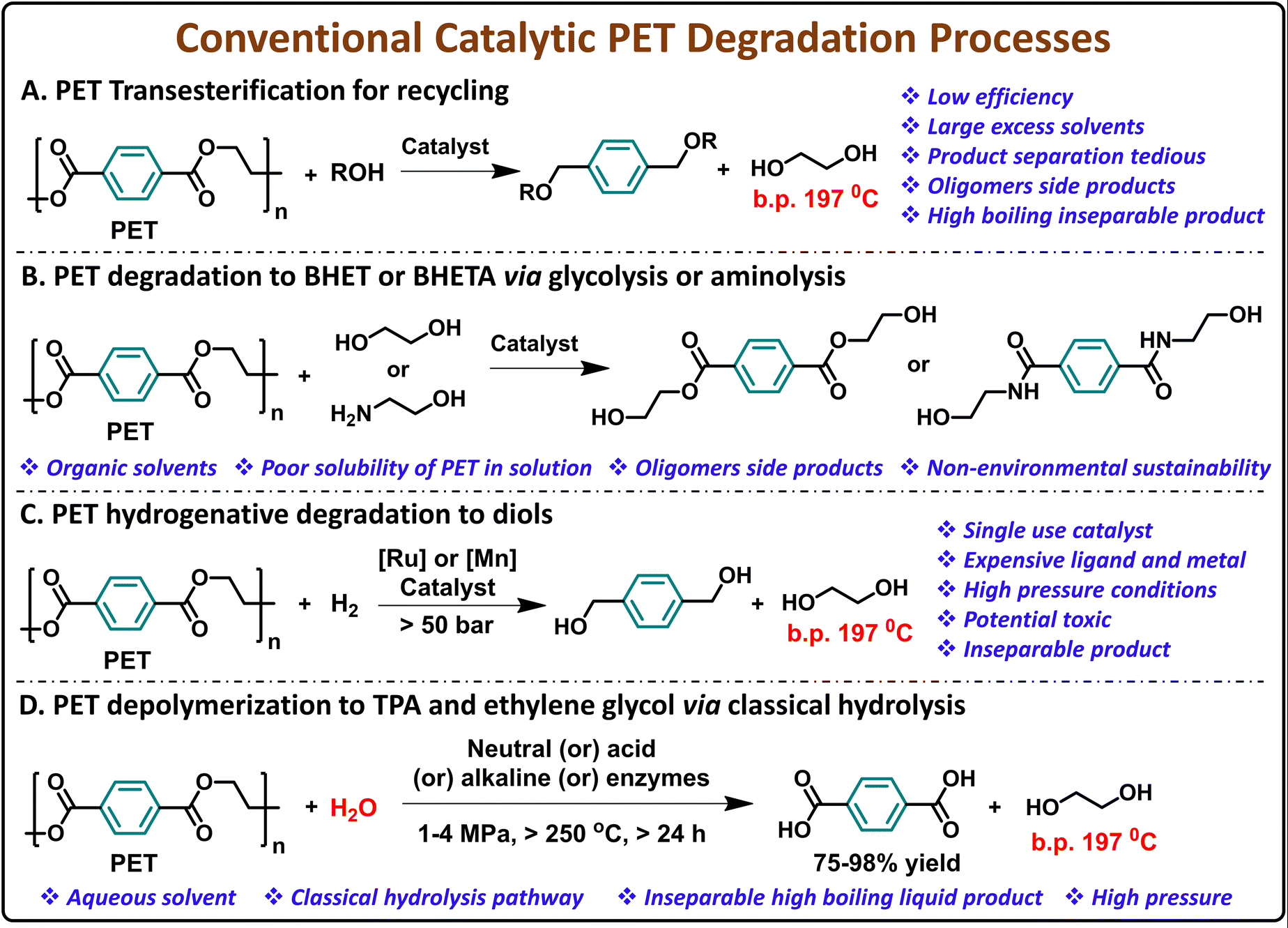
Recycling PET has traditionally relied on thermo-mechanical methods, which involve washing, shredding, and remelting the plastic. While this process is cost-effective, it degrades plastic quality over time, limiting its reuse. As a result, much of the plastic waste stream is deemed unrecyclable.
Chemical and enzymatic recycling methods have emerged as alternatives, breaking PET down into its building blocks for reuse. However, these methods require extreme conditions—high temperatures, high pressures, and toxic solvents—which make them costly and environmentally harmful.
Metal-based catalysts have been used to speed up chemical recycling, but they introduce new problems. Many of these catalysts are expensive, difficult to recover, and contribute to additional waste. Some methods use ionic liquids or deep-eutectic solvents, but these also pose environmental concerns. To solve these challenges, researchers have been searching for a greener, more efficient approach to PET recycling.
Related Stories
A research team at Northwestern University has developed a groundbreaking method to break down PET without toxic chemicals, excessive heat, or harsh solvents. Instead, their approach harnesses trace amounts of moisture in the air to selectively break down PET into its original monomers, which can then be recycled into new plastic or even upcycled into higher-value materials.
“The U.S. is the number one plastic polluter per capita, and we only recycle 5% of those plastics,” said Yosi Kratish, a lead researcher on the project. “There is a dire need for better technologies that can process different types of plastic waste. Most of today’s methods melt plastic bottles and downcycle them into lower-quality products. What’s particularly exciting about our research is that we harnessed moisture from air to break down plastics, achieving an exceptionally clean and selective process.”
The new process involves using a simple, earth-abundant molybdenum catalyst supported by activated carbon. The PET plastic is exposed to this catalyst and heated to 250-265°C, just above PET’s melting point. Once the plastic’s chemical bonds are weakened, exposure to air completes the breakdown process, converting PET into terephthalic acid (TPA), a valuable chemical used in making new plastics. Unlike traditional methods, this process produces almost no waste byproducts—only acetaldehyde, an easy-to-remove industrial chemical.
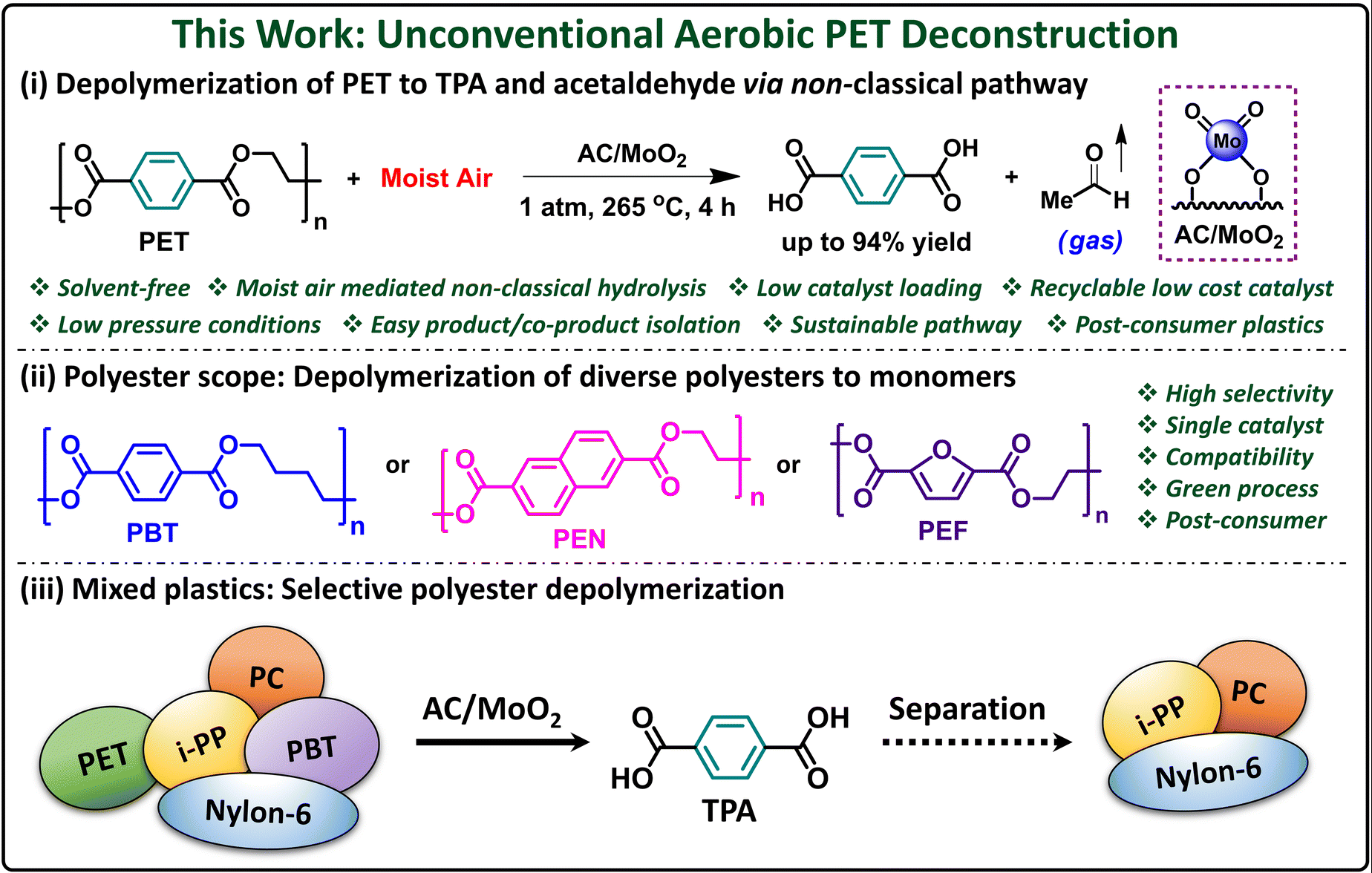
This method offers several advantages over existing recycling technologies. First, it eliminates the need for expensive and toxic solvents, reducing environmental impact. The reaction is also highly selective, meaning it only breaks down PET while leaving other plastics untouched. This eliminates the costly and time-consuming need to sort plastic waste before recycling.
“Using solvents has many disadvantages,” Kratish explained. “They can be expensive, and you have to heat them to high temperatures. Then, after the reaction, you’re left with a soup of materials that have to be sorted to recover the monomers. Instead of using solvents, we used water vapor from air. It’s a much more elegant way to tackle plastic recycling issues.”
The speed of the process is another major advantage. Traditional chemical recycling methods can take up to 72 hours, but the Northwestern approach achieves near-complete PET breakdown in just four hours. The catalyst itself is also durable and reusable, maintaining its effectiveness through multiple recycling cycles.
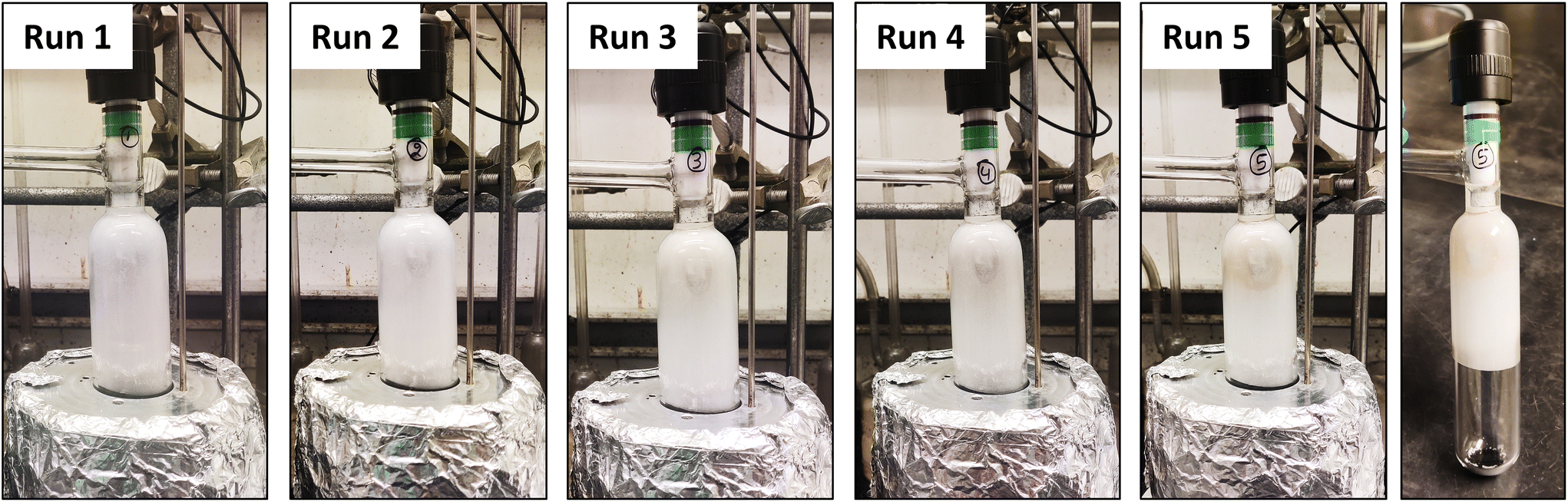
“The resulting process is fast and effective,” said Naveen Malik, the study’s first author. “In just four hours, 94% of the possible TPA was recovered. The catalyst is durable and recyclable, meaning it can be used time and time again without losing effectiveness.”
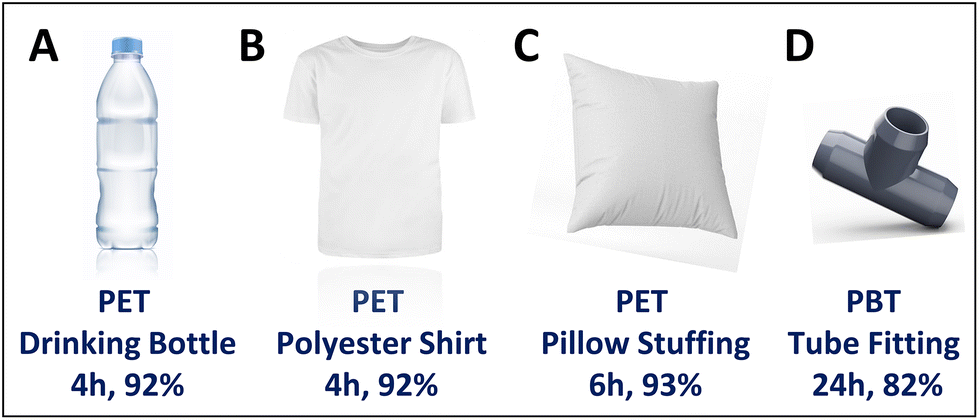
Additionally, the method works on real-world plastic waste. The researchers tested it on plastic bottles, polyester fabrics, and even mixed plastic waste, finding that it successfully extracted pure, colorless TPA from colored plastics as well. Unlike other methods that require pre-cleaned PET, this process works even on contaminated plastic waste.
The Northwestern team is now working on scaling up the process to handle industrial-scale plastic recycling. With further optimization, they believe this technology could significantly reduce global plastic pollution and contribute to a circular economy where plastics are continuously reused rather than discarded.
“Our technology has the potential to significantly reduce plastic pollution, lower the environmental footprint of plastics, and contribute to a circular economy where materials are reused rather than discarded,” Malik said. “It’s a tangible step toward a cleaner, greener future, and it demonstrates how innovative chemistry can address global challenges in a way that aligns with nature.”
If adopted on a large scale, this technology could help solve one of the world’s most pressing environmental challenges—plastic waste. By eliminating the need for harsh chemicals and excess energy, this method makes recycling more practical, cost-effective, and environmentally friendly.
With millions of tons of plastic waste piling up every year, innovations like this could play a key role in building a more sustainable future.
Research findings are published in the journal Royal Society of Chemistry.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post New technology uses air moisture to quickly recycle plastics with 94% efficiency appeared first on The Brighter Side of News.