Leptin, a hormone derived from adipose tissue, plays a central role in maintaining energy balance and controlling hunger. It sends signals to the brain to suppress food intake and regulate body weight.
Within the arcuate nucleus (ARC) of the hypothalamus, leptin influences two key neuronal populations. One, the agouti-related protein (AGRP) and neuropeptide Y (NPY) neurons, stimulates hunger, while the other, pro-opiomelanocortin (POMC) neurons, curbs it. This delicate balance orchestrates appetite regulation.
However, recent research has revealed a more complex picture. Although POMC and AGRP/NPY neurons are central to the regulation of food intake, their roles are not entirely reciprocal. AGRP/NPY neurons induce immediate food-seeking behavior and significantly impact body weight when disrupted.
In contrast, POMC neurons play a subtler role, with genetic mutations in POMC or its pathways leading to obesity, but their acute activation showing minimal effects on feeding.
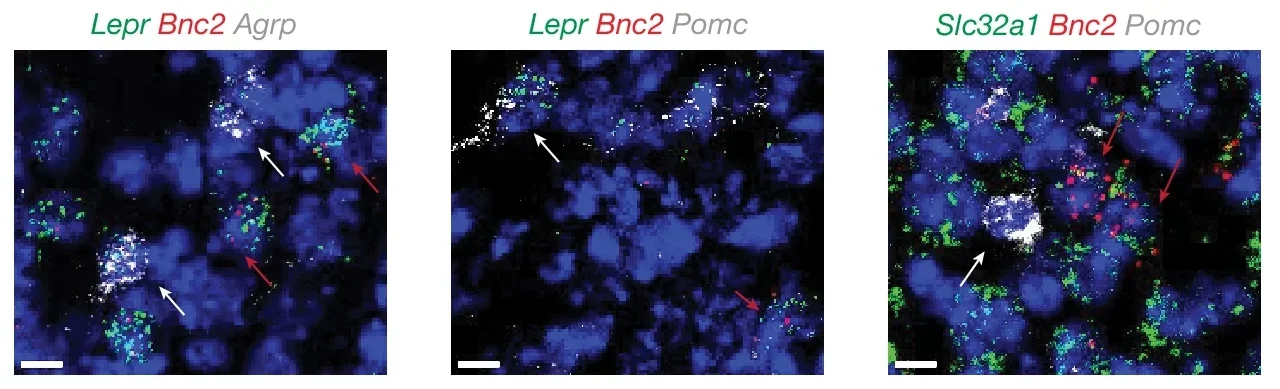
Leptin receptor (LepR) expression varies significantly between these neuron types. AGRP/NPY neurons, when lacking LepR, lead to severe obesity, while POMC neurons without LepR show only modest impacts.
This disparity suggests the existence of additional leptin-responsive neurons that contribute to appetite suppression and weight regulation. Emerging evidence points to a previously unrecognized neural population as the missing piece of this intricate puzzle.
In a groundbreaking study published in Nature, a team of researchers from the Laboratory of Molecular Genetics at Rockefeller University in New York, the Institute for Genome Science (IGS) at the University of Maryland School of Medicine (UMSOM) in Baltimore, as well as New York and Stanford Universities identified a novel group of neurons in the ARC that express both LepR and the gene basonuclin 2 (Bnc2).
Using single-nucleus RNA sequencing (snRNA-seq), the team uncovered these BNC2 neurons, which respond rapidly to leptin and induce satiety with kinetics mirroring the hunger-triggering effects of AGRP/NPY neurons. This discovery provides a fresh perspective on how leptin regulates energy balance and offers potential new strategies for combating obesity.
Related Stories
The urgency of this research is underscored by the staggering prevalence of obesity, which affects 40% of adults and 20% of children in the United States. Although therapies such as semaglutide have shown promise, a deeper understanding of the brain-body connection that governs hunger and weight is essential for developing more effective interventions.
Brian Herb, PhD, a scientist at the University of Maryland School of Medicine, emphasized the importance of this discovery in light of previous work mapping neuronal populations in the human hypothalamus. “We’ve long known that the hypothalamus plays a role in hunger, hormone levels, stress responses, and body temperature,” he explained. “Our earlier research demonstrated that unique genetic programs give rise to specialized neurons. It makes sense that we’re now uncovering a previously unknown set of neurons that regulate energy and food intake.”
The researchers conducted experiments with mice to explore the functions of BNC2 neurons further. They used CRISPR-Cas9 technology to knock out LepR in these neurons. The modified mice exhibited increased food consumption and significant weight gain compared to control groups.
Additionally, the team observed that after a fasting period, BNC2 neurons became highly active in response to feeding, whereas other hypothalamic neurons remained unresponsive. This behavior highlights their role in responding to food-related sensory cues, such as taste and nutritional content.
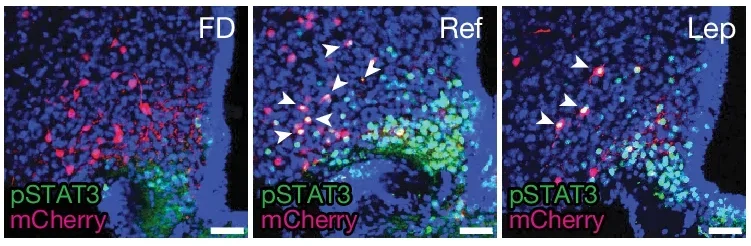
The implications of these findings extend beyond basic neuroscience. Activating BNC2 neurons could become a novel therapeutic strategy for obesity.
Dr. Herb noted, “These findings add a critical new component to our understanding of how neurons impact appetite and obesity. This could be a future target for obesity treatments aimed at suppressing hunger or reducing weight.”
Despite these promising discoveries, challenges remain. While AGRP/NPY and POMC neurons have been studied extensively, their roles are nuanced and context-dependent. AGRP/NPY neurons, for example, are associated with negative emotions during hunger, while POMC activation does not elicit positive emotions.
Additionally, AGRP/NPY neurons influence glucose metabolism, a function not shared by POMC neurons, indicating that their effects on the body extend beyond appetite.
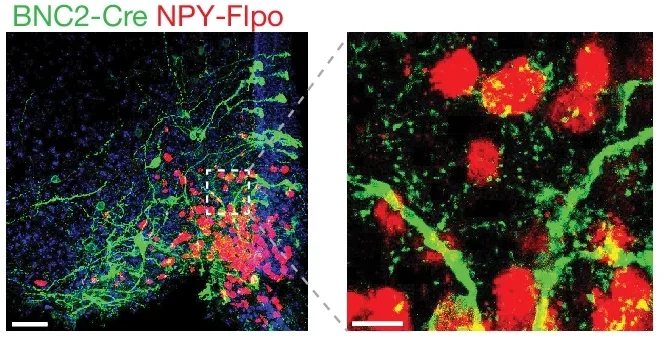
The identification of BNC2 neurons adds another layer of complexity. These neurons demonstrate that leptin’s role in energy balance is not confined to the previously known populations in the hypothalamus. The discovery also underscores the power of advanced techniques like snRNA-seq in uncovering new cellular players in biological systems.
This breakthrough marks a significant step forward in understanding the neural mechanisms underlying hunger and satiety. As obesity rates continue to climb, the need for innovative approaches to treatment becomes more pressing.
By targeting BNC2 neurons or developing drugs that mimic their activity, researchers may one day offer new hope to millions struggling with weight-related health issues.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Newly discovered neurons could revolutionize obesity treatment appeared first on The Brighter Side of News.