Surface-enhanced Raman spectroscopy (SERS) is revolutionizing biomedical diagnostics and therapeutics, enabling breakthroughs in biopharmaceuticals, antimicrobial resistance detection, forensic science, and homeland security. With its unique ability to detect molecular fingerprints with single-molecule sensitivity,
SERS offers unparalleled potential. However, translating this method into reliable clinical tools faces significant hurdles—chiefly the reliance on electric fields for signal enhancement and unpredictable intensity fluctuations.
Traditionally, SERS enhancement depends on electromagnetic fields, particularly electric fields (E-fields). While effective, the magnetic field (H-field) component remains underutilized due to the limited magnetic permeability of metals at optical frequencies.
This omission underscores a missed opportunity to fully exploit electromagnetic mechanisms in SERS. Addressing this gap requires innovative solutions, such as plasmonic substrates that activate both E- and H-fields.
Recent advances in photonic metamaterials present a promising path forward. Published in the journal, Science Advances, these engineered structures exhibit unique optical properties, including the ability to generate significant magnetism at optical frequencies.

For example, split-ring resonators and layered hybrid metal-dielectric nanostructures have shown potential in creating denser and more consistent SERS hotspots by simultaneously activating E- and H-fields.
The complementary relationship between these fields mitigates the spatial heterogeneity of SERS hotspots, addressing one of the primary causes of intensity fluctuations. Such advancements enhance the reliability of SERS-based diagnostics, laying the groundwork for widespread clinical adoption.
The innovation in using metamaterials extends beyond mere signal enhancement. By carefully designing the nanoscale structures of these materials, researchers can control the distribution of electromagnetic fields with precision.
This control ensures that both electric and magnetic field components are optimized for maximum Raman signal amplification. Furthermore, the ability to fine-tune these properties allows for the development of SERS platforms tailored to specific diagnostic applications, from detecting infectious diseases to monitoring environmental toxins.
While intensity-based SERS methods often face challenges like molecular blinking and dynamic interactions, frequency-based detection offers a robust alternative. The vibrational frequencies of molecules, which correspond to specific Raman peaks, remain stable despite variations in signal intensity. This stability enables more reliable diagnostic applications.
Related Stories
Research has demonstrated the viability of frequency-based SERS immunoassays using antibody-antigen interactions. These interactions induce structural changes in antibody-conjugated Raman molecules, resulting in characteristic frequency shifts.
Notably, this method simplifies assay design by requiring only one type of antibody, reducing time, cost, and complexity. Such frequency-based approaches have successfully detected proteins, nucleic acids, and even circulating tumor DNA.
The adoption of frequency-based SERS has implications beyond simplifying assay procedures. It addresses one of the long-standing challenges in spectroscopic analysis: reproducibility.
By focusing on frequency shifts rather than intensity variations, researchers can achieve more consistent and reliable results, even in complex biological environments. This advancement is particularly important for applications in personalized medicine, where diagnostic precision can significantly impact treatment outcomes.
Building on these advances, researchers have combined plasmonic metasurfaces and frequency-shift monitoring to develop a groundbreaking diagnostic tool for cardiac biomarkers.
Acute myocardial infarction (AMI), commonly known as a heart attack, requires rapid diagnosis to enable timely medical intervention. Key biomarkers for AMI include creatine kinase-myocardial band (CK-MB), myoglobin (Mb), and cardiac troponin-I (cTnI).
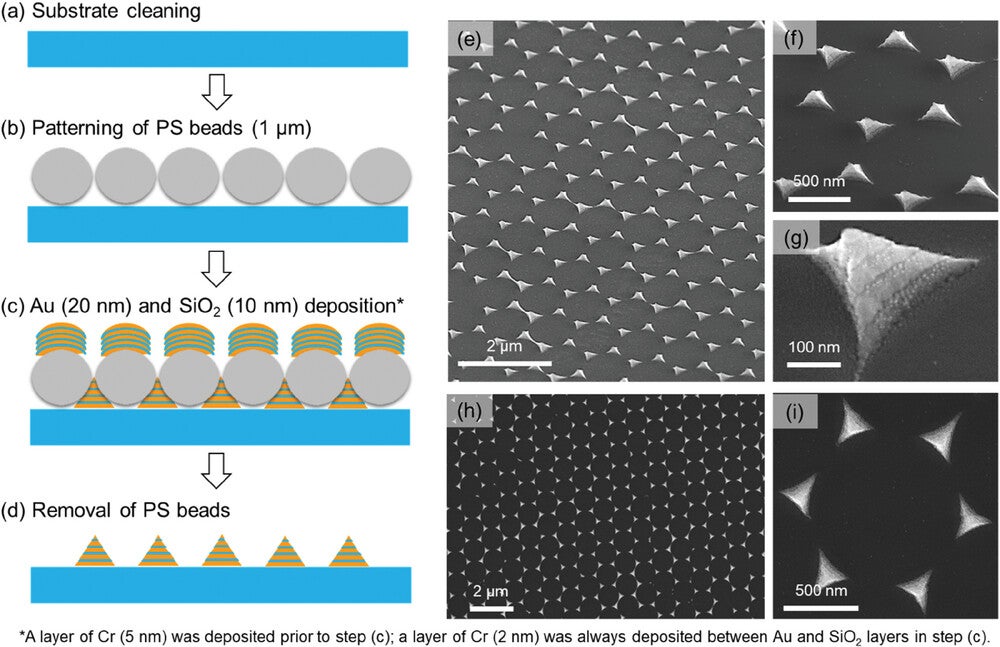
This new diagnostic platform employs alternately stacked gold-silica pyramidal metasurfaces. These structures, fabricated using nanosphere lithography, activate both E- and H-fields to enhance SERS signals. Complemented by 3D-printed compartmentalized biosensors, the platform allows simultaneous detection of multiple biomarkers with unprecedented speed and accuracy.
Finite-difference time-domain simulations confirmed the metasurfaces’ ability to produce spatially consistent SERS enhancement. By addressing the “SERS memory effect”—a common challenge in reusing substrates—this method enables high-throughput, interference-free analysis of serum samples. The result is a stand-alone blood test capable of delivering results in five to seven minutes, outperforming traditional methods that can take hours.
The ability to detect multiple biomarkers simultaneously is a significant advancement. Current diagnostic methods often rely on separate tests for each biomarker, increasing the time and cost of diagnosis.
The integration of multiplexed detection into a single platform not only streamlines the diagnostic process but also reduces the likelihood of errors associated with handling multiple samples. This improvement is particularly valuable in emergency settings, where rapid and accurate diagnosis can be lifesaving.
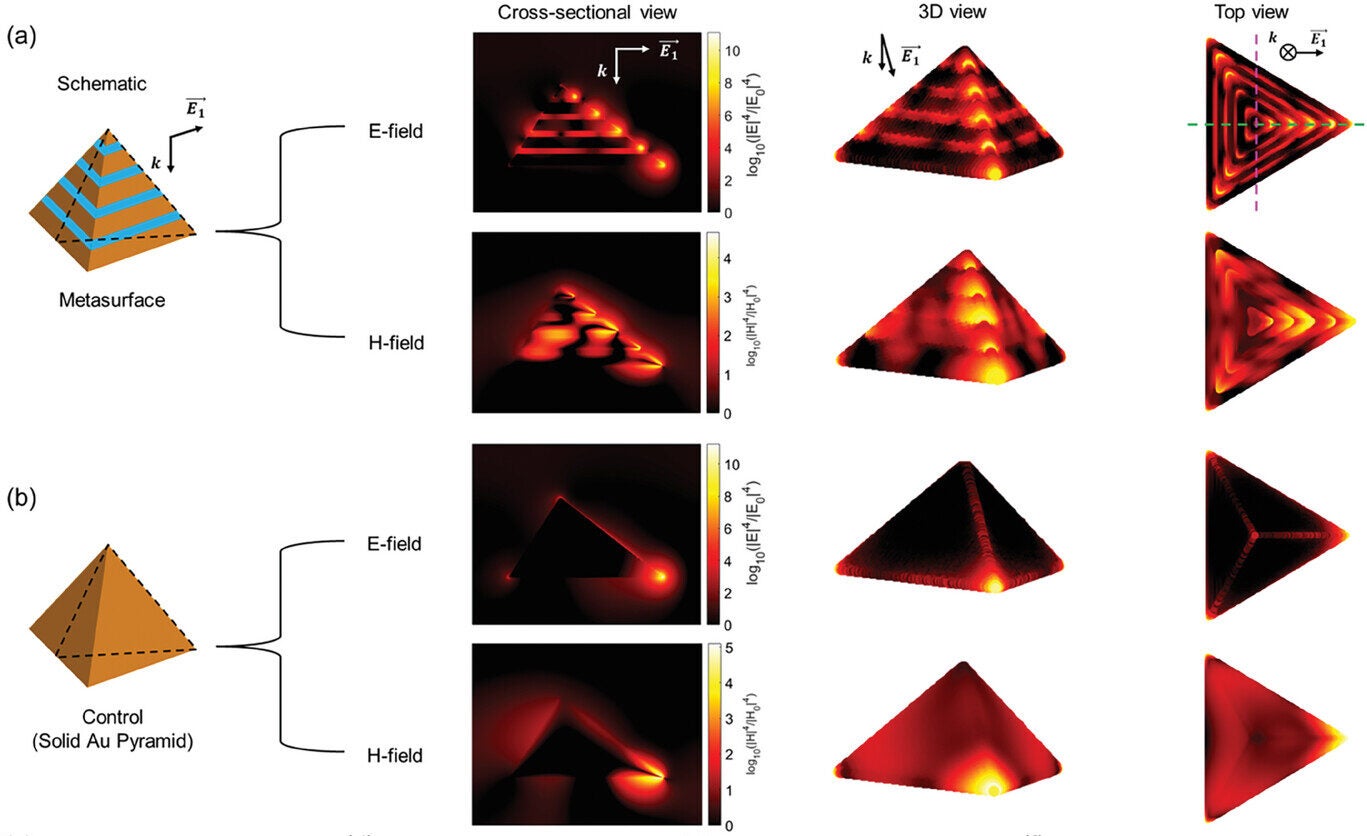
The heart of this innovation lies in its chip-based design, featuring a nanostructured metasurface. This surface amplifies both electric and magnetic signals during Raman spectroscopy, making it sensitive enough to detect ultra-low concentrations of biomarkers. This capability is crucial for early-stage diagnosis when medical intervention can save lives.
Beyond its clinical applications, the technology holds promise for first responders and even at-home use. A handheld device based on this platform could enable rapid, accurate diagnostics outside hospital settings. “We’re talking about speed, accuracy, and the ability to perform measurements outside of a hospital,” explained bioengineer Ishan Barman. Such advancements could revolutionize emergency care and democratize access to critical diagnostic tools.
The potential for at-home diagnostic tools represents a major shift in healthcare delivery. By empowering individuals to monitor their health in real time, these devices could reduce the burden on healthcare systems and enable earlier detection of conditions that might otherwise go unnoticed. This approach aligns with the growing emphasis on preventative medicine and personalized healthcare.
While initially designed for heart attack diagnosis, this platform technology has broader implications. It could be adapted to detect cancer biomarkers, infectious diseases, and other health conditions. The flexibility of the metasurface design makes it a versatile tool for various biomedical applications.
“There is enormous commercial potential,” said Barman. “There’s nothing that limits this platform technology.” Future research will focus on refining the test and conducting larger clinical trials to validate its efficacy across diverse populations and conditions.
The development of this technology also opens the door to new research opportunities in other fields. For example, environmental scientists could use similar platforms to detect pollutants at trace levels, while forensic experts might employ them to analyze crime scene evidence with unprecedented precision. These interdisciplinary applications highlight the versatility and far-reaching impact of advances in SERS-based diagnostics.
This innovation exemplifies the transformative power of combining advanced materials science with biophotonics. By leveraging the strengths of both E- and H-fields and embracing frequency-based detection, SERS is poised to redefine diagnostics.
From improving heart attack outcomes to enabling early detection of diseases, this technology heralds a new era of precision medicine.
The integration of advanced spectroscopy, innovative materials, and cutting-edge engineering underscores the potential for SERS to address some of the most pressing challenges in healthcare and beyond.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Revolutionary nano-chip quickly detects earliest sign of heart attack appeared first on The Brighter Side of News.