Scientists at NYU Abu Dhabi have developed an innovative use for nanotechnology in the field of cancer research. Their multidisciplinary team, led by Biology Associate Professor Mazin Magzoub, is focused on utilizing this technology to enhance the precision of cancer treatment while minimizing damage to the surrounding healthy tissue.
Their work focuses on new developments in the use of light in Photothermal Therapy (PTT), which uses heat generated by light to kill cancerous tumor cells. Traditional methods used to treat cancer are chemotherapy and radiation. However, PTT offers potential ways to directly target tumor cells while also eliminating the collateral damage that occurs when using chemotherapy or radiation treatments.
To build a targeted approach to PTT, the NYU Abu Dhabi research team designed very small nanoparticles responsive to near-infrared (NIR) electromagnetic (EM) radiation, enabling the clinician to heat the tumor from within. Additionally, the research team designed and synthesized nanoparticles capable of delivering heat directly to the tumor cells.
NIR light is ideal for this type of application because it penetrates the tissues in the body more effectively than visible EM radiation. This allows access to tumors that reside deep beneath the outer surface of the skin. One of the main challenges the team encountered was developing materials that remain stable inside the body long enough to penetrate deep enough to effectively deliver the PTT treatment.
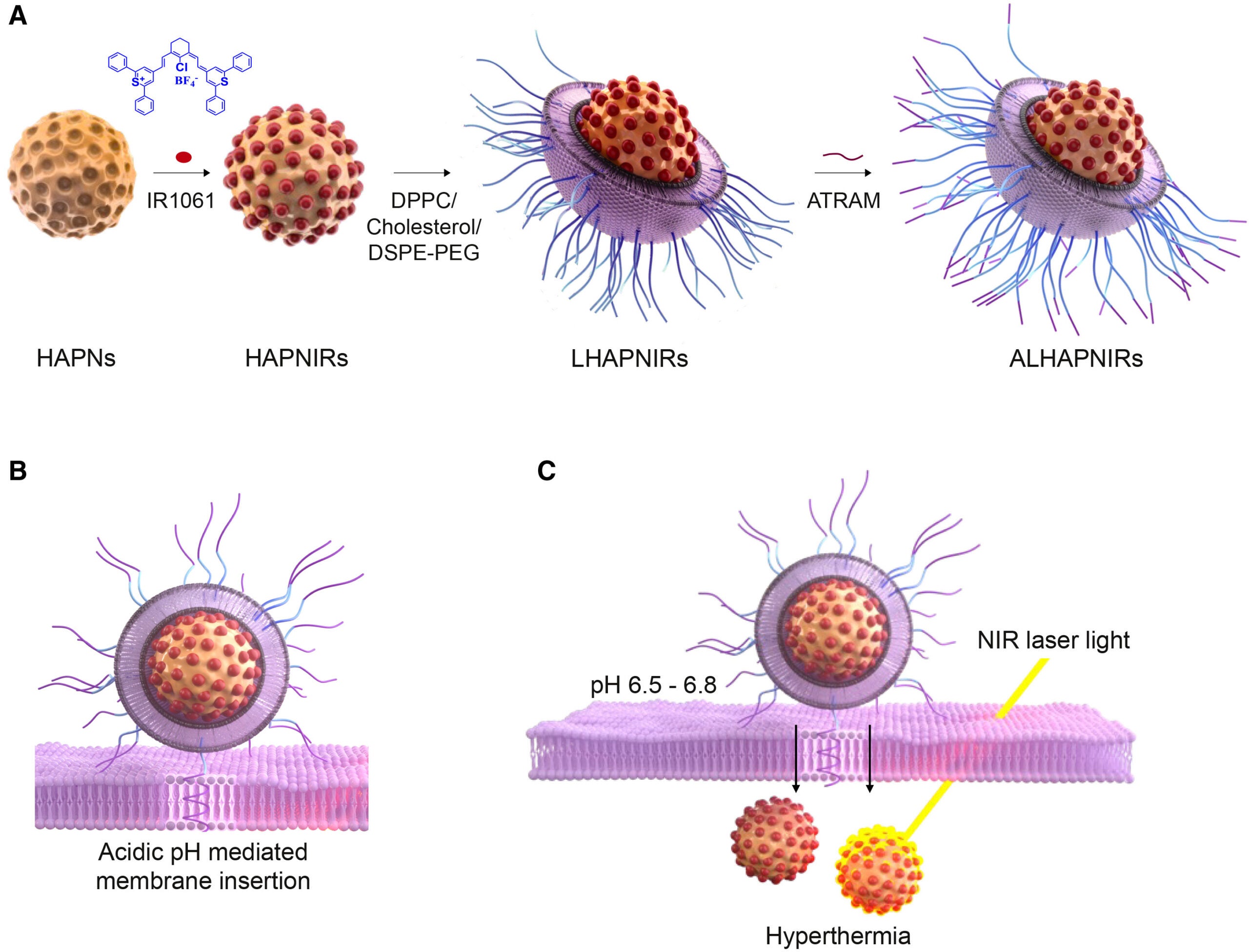
To address these issues, the research team chose to use hydroxyapatite (HA) as an ideal starting point for the new nanoparticles. This mineral is already produced and stored in our bones and teeth, which means that our body perceives it as a naturally occurring and harmless substance. In addition to biocompatibility, HA is also biodegradable, which means that it will be broken down and eliminated from our bodies naturally.
The research team created spherical nanoparticles from this mineral with very small pores that serve to encapsulate a light-sensitive dye. In order to improve the stability of these NIR-emitting nanoparticles, the research team applied a thin coating of lipids and polymers to the surface of each particle to increase structural integrity and bioavailability. This coating extends the circulation time of nanoparticles within the vascular system and diminishes the likelihood of detection by the host organism’s immune response, allowing for more nanoparticles to arrive at their tumor destinations.
Researchers also applied a peptide called ATRAM that reacts with acidic conditions to assist the attached nanoparticles in entering the tumor environment. Solid tumors are typically located in an environment where the pH is slightly below neutral compared to surrounding non-neoplastic tissues. The ATRAM peptide is activated in an acidic environment, allowing it to bind to and enter tumor cells.
According to Magzoub: “This work provides a comprehensive solution for targeted therapy and imaging through one biocompatible and biodegradable delivery system. We believe that addressing major obstacles associated with delivering treatment to tumors will ultimately help to enhance the precision of cancer therapies.”
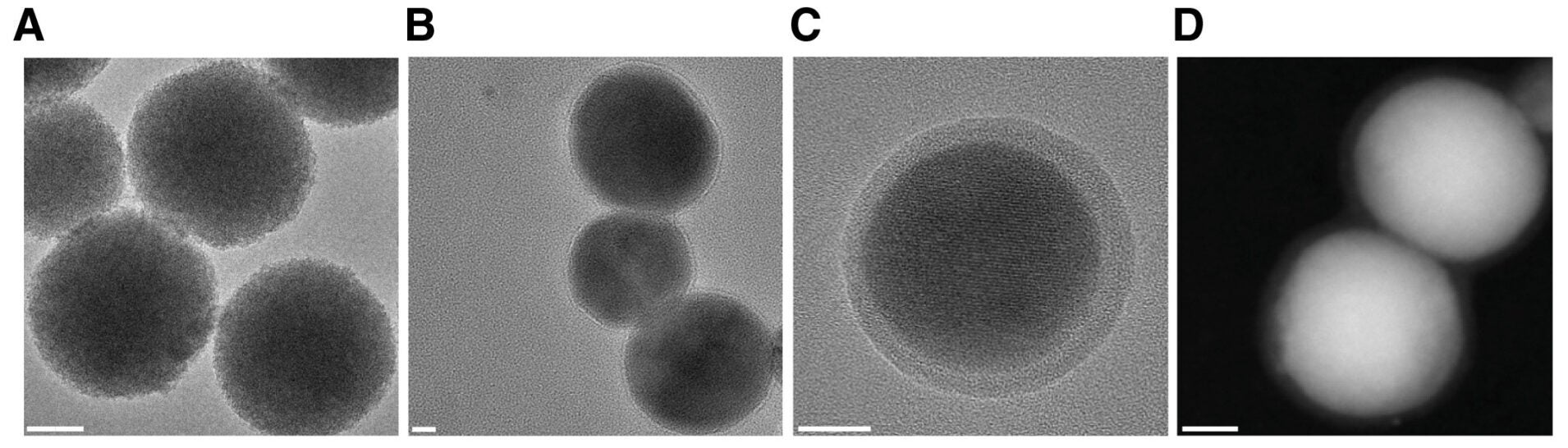
Upon entering tumor cells via the nanoparticles, doctors will be able to ascertain where tumor cells are located due to fluorescent emission from the dye contained within the nanoparticles and to track these cells in real time. Consequently, when exposed to light in the near-infrared spectrum, the dye within the nanoparticles will convert this light into heat.
In laboratory analyses, researchers have determined that the quantity of heat produced by the nanoparticles depends upon both the concentration of nanoparticles and the intensity of the light. In certain cases, this heat was sufficient to kill malignant cells. The stability of the heating effects of the nanoparticles after exposure to light indicates that they do not degrade rapidly.
“The nanoparticles enhanced the performance of the dye by encapsulating it within the hydroxyapatite, thereby providing an extra level of protection against degradation. This allowed for a greater heat-to-light conversion efficiency,” Magzoub told The Brighter Side of News.
“The nanoparticles were tested on human pancreatic cancer cells grown in a laboratory environment. When the pH of the system was at the physiological range, the uptake by the cells was very low and caused minimal harm when illuminated. The uptake significantly increased when the pH of the system was lowered to levels that are similar to those found in tumors,” he added.
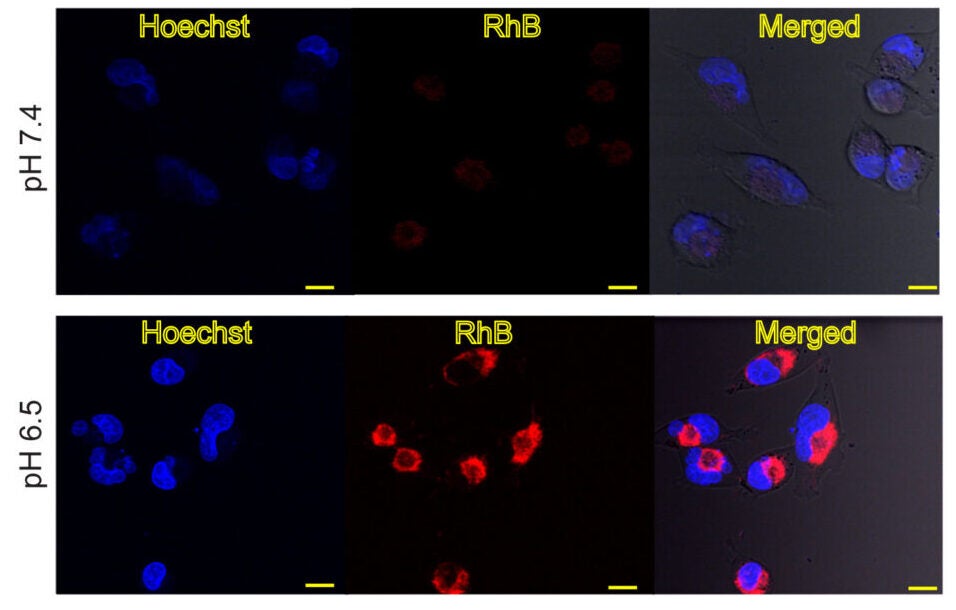
In the presence of near-infrared light and the lower pH, there was a significant decrease in the survival rate of the cancer cells. Healthy cells did not exhibit the same response. Further analyses determined that many of the cancer cells died through apoptosis, which is a programmed method of necrosis that occurs due to thermal ablation.
This indicates that the nanoparticles were capable of selectively damaging or killing cancer cells without adversely affecting surrounding healthy tissue.
The in vivo testing of the nanoparticles was conducted on mice with pancreatic tumors. The nanoparticles were injected intravenously and accumulated in the tumor mass more so than in any of the large organs. For several days post-administration of the dye, a signal from the tumor was visible, which allowed the researchers to track the nanoparticles.
In the presence of near-infrared light, the nanoparticles were heated to much higher temperatures than the simple particles, and the nanoparticles heated up more rapidly. With the addition of time, the mice that received the nanoparticles and were treated with near-infrared light had a more substantial decrease in tumor volume. They lived longer than those that were not treated with nanoparticles and were less affected than all other groups.
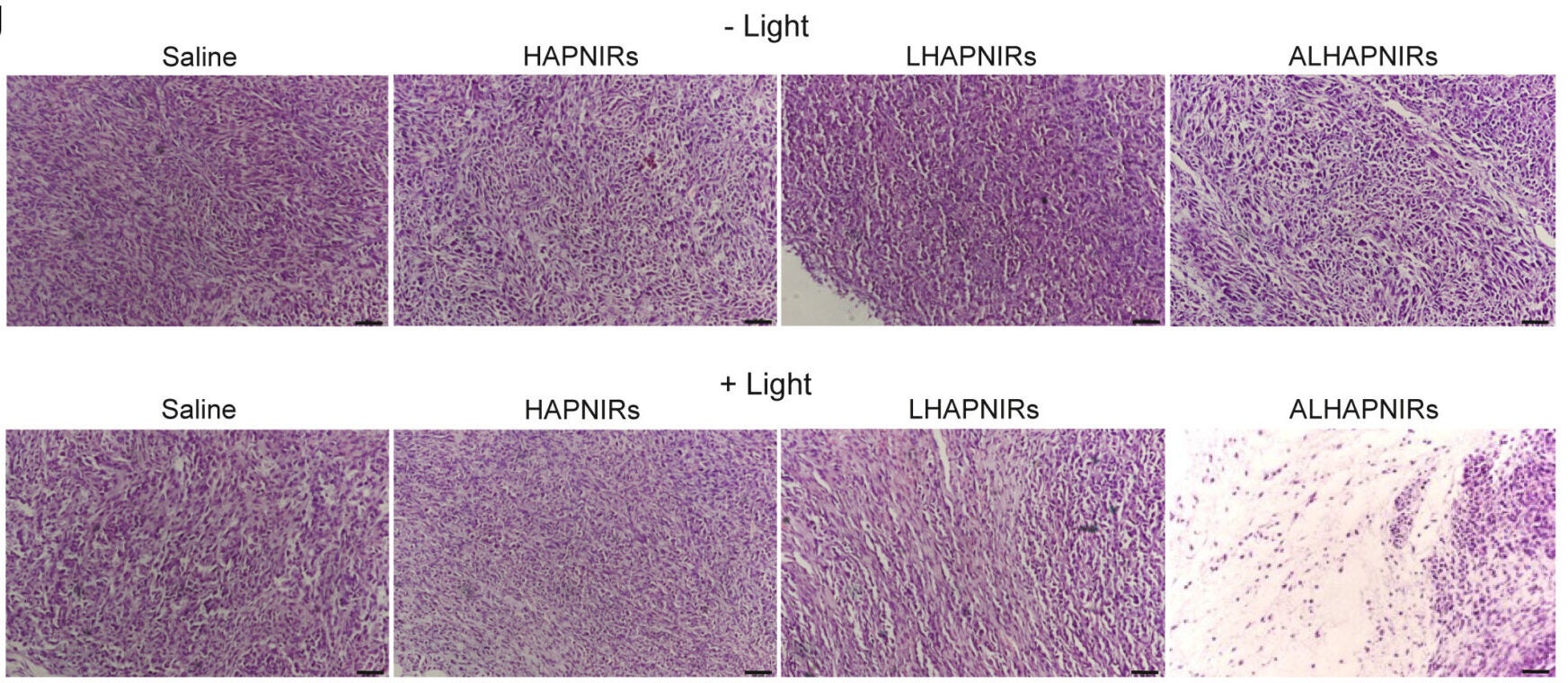
No significant adverse effects were experienced by the mice during safety testing. The mice’s body weights were stable, their organs were healthy as determined by microscopic examination, and their blood tests exhibited no evidence of inflammation or organ failure. The dye was eliminated from the mouse’s systemic circulation within approximately three days.
The data from this study supports the prospect of developing more selective and less damaging cancer therapies in the future by integrating both diagnostic imaging and therapeutic capabilities into one platform. This approach allows a physician to detect tumors, monitor their progression, and effectuate the destruction of malignant cells simultaneously.
If this approach translates effectively into human clinical applications, the reliance on traditional and aggressive cancer therapies like chemotherapy and radiation may be lessened. Additional treatment avenues may also be made available for treating tumors that would otherwise be inaccessible surgically.
The approach has the potential to stimulate additional innovations in drug delivery systems and consequently enhance the discipline of precision medicine within many fields.
Research findings are available online in the journal Cell Reports Physical Science.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Scientists create light-activated nanoparticles that safely eradicate tumors and cancer cells appeared first on The Brighter Side of News.