Cells constantly shift and transform, triggering the complex choreography that shapes living organisms. Whether dividing into new cells or sculpting an embryo, these tiny movements rely on chemical signals coordinating precise mechanical reactions. Recently, scientists harnessed these movements using beams of light to control cellular behavior, taking a big step toward creating programmable synthetic cells.
Every living thing—from bacteria to humans—depends on cells dynamically changing shape. These tiny shape shifts allow cells to divide, move, and build tissues, forming complete organisms.
Behind each transformation is an intricate system of chemical signals interacting with tiny muscle-like fibers inside the cell. This powerful combination, called chemo-mechanical signaling, drives critical biological processes, including wound healing and embryo development.
However, scientists face challenges in fully understanding these processes because they involve complex chemical interactions. To study these reactions accurately, researchers need precise control over how and when the cell receives these chemical signals. That’s why scientists have turned to a powerful new tool: light.

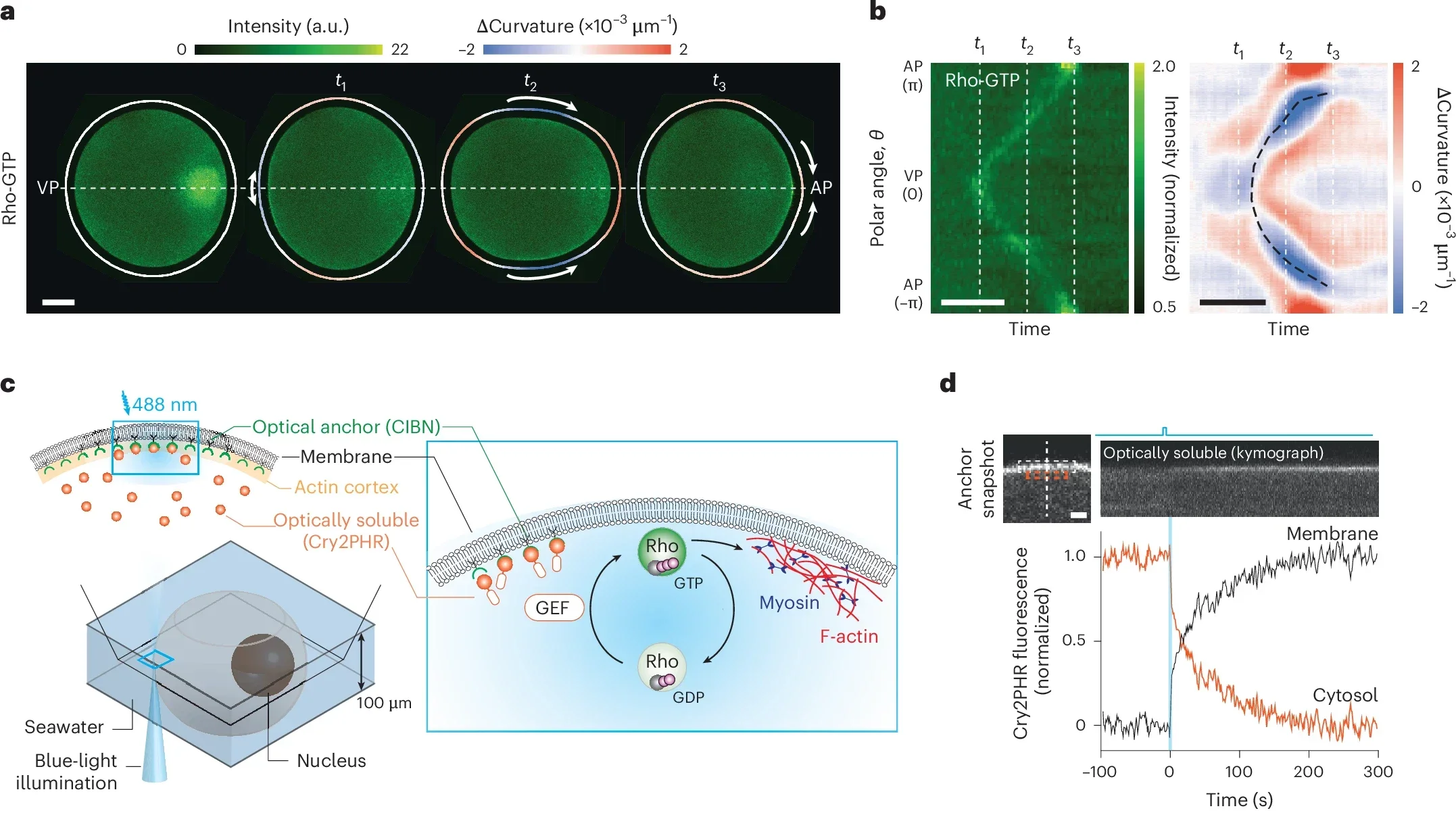
A team of researchers led by physicist Nikta Fakhri has now used optogenetics—a method of controlling biological processes with light—to manipulate how cells move and reshape themselves. Specifically, they studied egg cells from starfish, organisms frequently used as models for cell growth and development research. Starfish eggs exhibit a unique phenomenon known as surface contraction waves, a form of cellular motion crucial during the egg’s early division process.
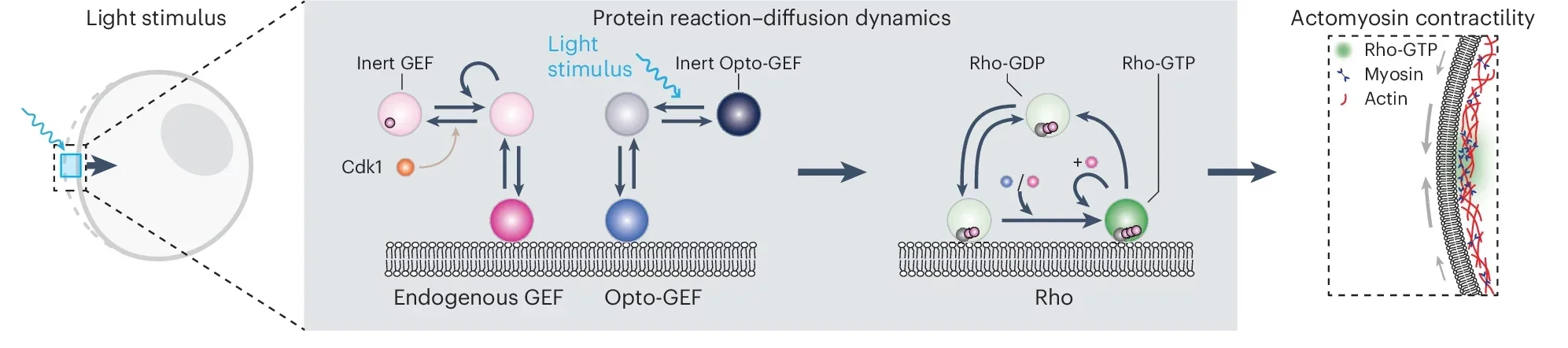
Normally, these waves are triggered by proteins called Rho and enzymes known as GEF, which together activate muscle-like contractions in the cell’s outer layer. When GEF stimulates Rho, the protein moves from the cell’s interior and binds to its surface membrane. This binding causes tiny fibers within the membrane to contract, changing the cell’s shape.
Researchers engineered a new type of GEF enzyme sensitive to light, giving them precise control over this cellular machinery. They extracted genetic instructions (mRNA) for the modified enzyme and injected these instructions into starfish egg cells. The cells began producing the new enzyme themselves, effectively becoming responsive to light signals.
Related Stories
Under a microscope, scientists then aimed tiny beams of light onto specific regions of these cells. As they did this, the light-sensitive GEF activated Rho proteins exactly where the light shone. Immediately, the activated Rho proteins triggered contractions in the cell surface, causing predictable and precise shape changes.
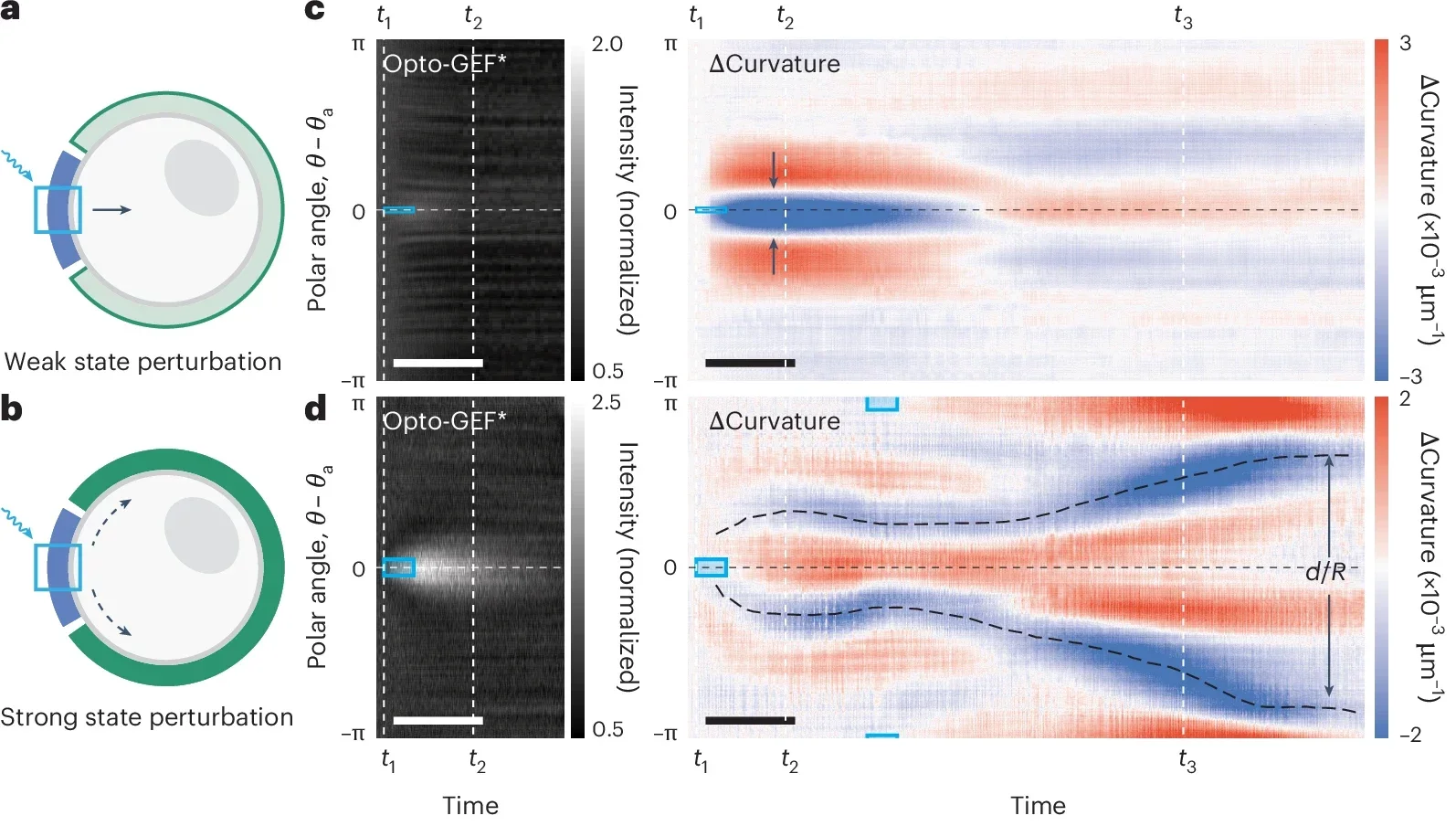
The researchers were able to induce small, local pinches or larger, sweeping waves of contraction. Remarkably, by carefully placing beams of light around the cells, they even reshaped their outlines entirely—transforming normally round cells into distinctly square shapes. Such precision highlights the potential of optogenetics in guiding complex cellular movements.
“We realized this Rho-GEF circuitry is an excitable system, where a small, well-timed stimulus can trigger a large, all-or-nothing response,” said Nikta Fakhri, associate professor of physics at MIT and senior author of the study. “So we can either illuminate the whole cell or just a tiny place on the cell, recruiting enough enzyme so the system kickstarts contractions on its own.”
The team didn’t stop at manipulating the cells. They developed a mathematical model to predict how the cells would respond based on the pattern of light applied. This model accounts for chemical signals and how the cell’s geometry affects its movements. Through this framework, scientists can now predict the range of possible shapes and behaviors a cell might exhibit, based purely on how it’s stimulated.
This new model clarifies a fundamental aspect of cell biology: the ‘excitability’ of cells, which dictates their readiness to respond dramatically to minor signals. According to Fakhri, understanding this excitability can provide crucial insights into biological processes such as wound healing and embryo formation, where cell movements must be precisely timed and coordinated.
The broader implications of this research are significant. By mastering how cells move in response to chemical signals—now controlled precisely by light—scientists envision developing synthetic cells that perform specific medical functions. These could include therapeutic cells designed to contract when exposed to light, aiding in wound closure, or carrier cells programmed to deliver drugs exactly where and when needed inside the body.
“By revealing how a light-activated switch can reshape cells in real time, we’re uncovering basic design principles for how living systems self-organize and evolve shape,” said Fakhri. “The power of these tools is that they’re guiding us to decode all these processes of growth and development, helping us understand how nature does it.”
Starfish offer a uniquely useful model for studying cell growth and shape. These creatures begin life as symmetrical cells but develop complex symmetry patterns as they mature—from bilateral symmetry as larvae to five-fold symmetry as adults. Understanding the chemical signals guiding these symmetry changes provides essential clues about how living organisms organize themselves.
The researchers used starfish egg cells because these cells clearly demonstrate chemo-mechanical waves during division. These waves emerge due to peaks in the Rho protein, driven by the enzyme GEF. By choosing starfish cells, the researchers effectively showcased the potential to control and harness these fundamental biological processes in a simpler, observable system.
Advances in optogenetics bridge biology with technology, offering powerful tools to control life processes. Fakhri’s group’s achievement demonstrates that precise manipulation of cell behavior using light isn’t just theoretically interesting—it is now practical and achievable. By controlling the cell’s chemical circuitry with simple beams of light, scientists have created a groundbreaking way to influence how life physically develops.
The next steps include using this approach to create synthetic cells capable of complex, life-like movements. Future applications may revolutionize biotechnology, medicine, and materials science, inspiring innovations in artificial tissues, advanced wound healing therapies, and highly targeted drug delivery systems.
In uncovering the hidden choreography of cells, these researchers illuminate a pathway toward building programmable, life-like cells. Such advances hold promise not just in understanding biology but in applying that knowledge to enhance human health and technology, reshaping the boundaries between natural life and synthetic innovation.
The research findings were published in the journal Nature Physics.
Note: The article above provided above by The Brighter Side of News.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Scientists create synthetic cells that shape-shift in response to light appeared first on The Brighter Side of News.