Recent advances in molecular biology have provided unprecedented insights into the structure of amyloid fibrils associated with Huntington’s disease (HD).
By integrating experimental methods such as cryogenic electron microscopy (cryo-EM) and solid-state nuclear magnetic resonance (ssNMR), researchers are unraveling the complex architecture of protein aggregates that contribute to neurodegenerative disorders. These findings are essential for developing new diagnostic tools and potential treatments for this incurable disease.
Huntington’s disease arises from a genetic mutation that results in an abnormal expansion of the CAG trinucleotide sequence in the huntingtin (HTT) gene. This mutation causes the huntingtin protein to develop a polyglutamine (polyQ) tract that aggregates into amyloid fibrils. Unlike the fibrils associated with Alzheimer’s or Parkinson’s diseases, those formed by polyQ proteins present unique structural challenges.
PolyQ segments lack stable secondary structures in their native state, existing as intrinsically disordered regions. However, during disease progression, proteolytic cleavage and aberrant splicing of HTT generate N-terminal fragments containing polyQ expansions.
These fragments aggregate into amyloid fibrils, forming cellular inclusions that are hallmarks of HD pathology. The propensity of polyQ proteins to aggregate correlates with tract length, which inversely relates to the age of disease onset.

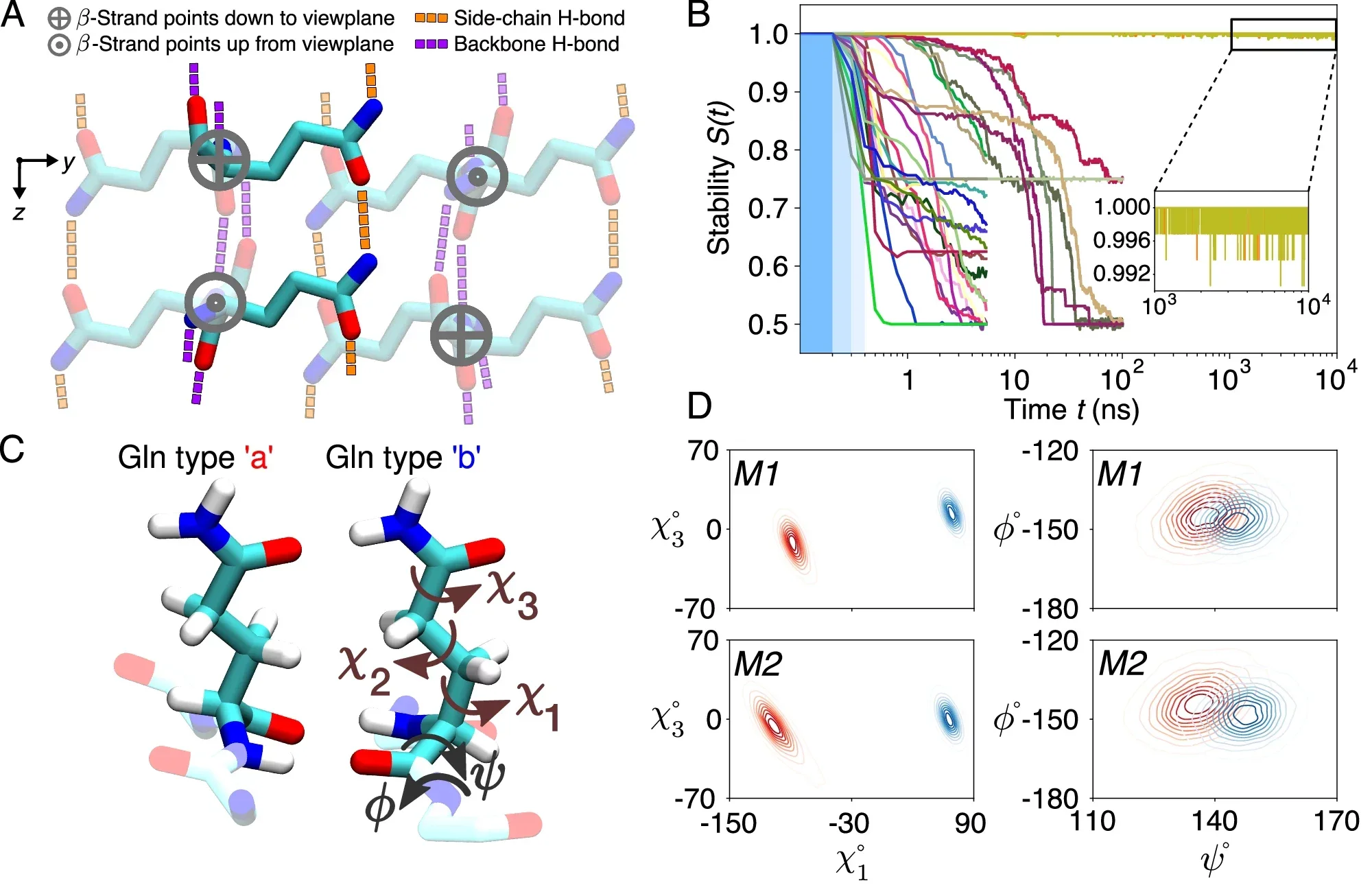
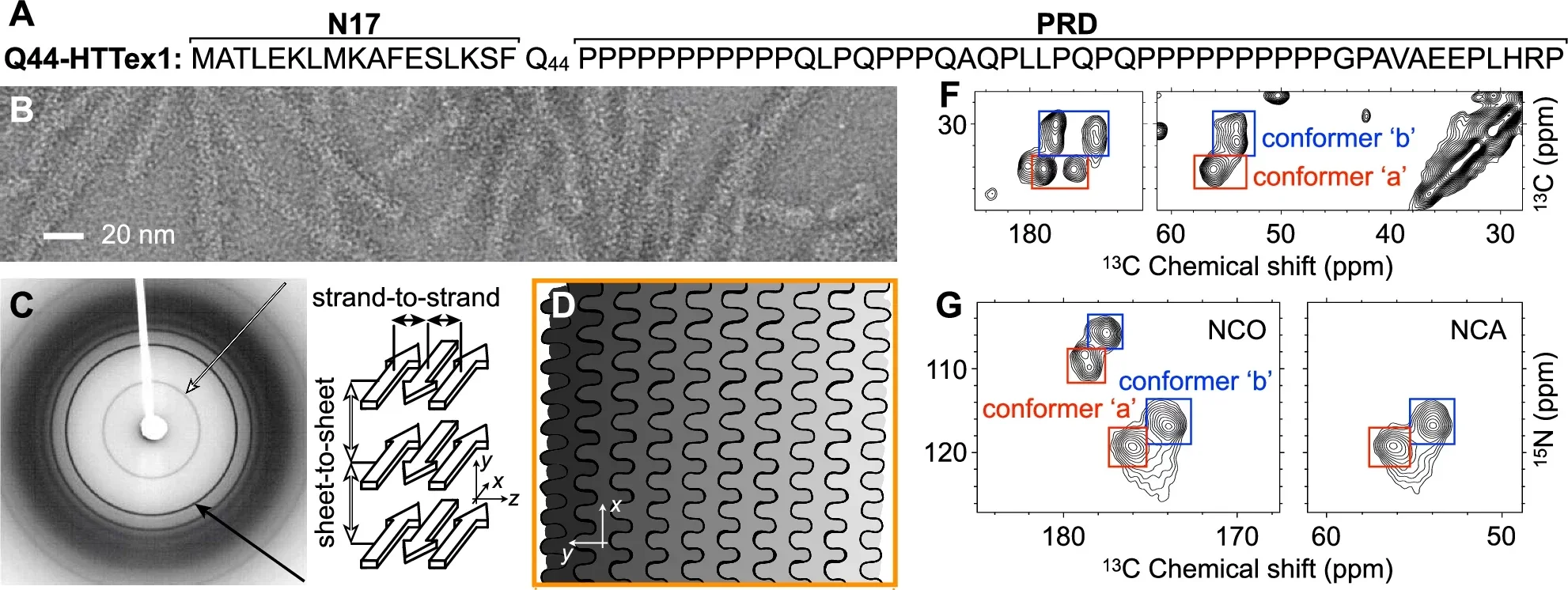
Despite extensive research, the atomic-level structure of polyQ fibrils has remained elusive, primarily due to their repetitive sequences and distinct aggregation patterns. These fibrils differ from other amyloids by featuring antiparallel β-sheets and block-like cores, making them particularly challenging to study using traditional techniques. Researchers have long sought to overcome these barriers to unlock insights critical to understanding and treating HD.
To address these challenges, a team of international researchers led by Markus Miettinen at the University of Bergen employed integrative structural biology.
By combining data from ssNMR, cryo-EM, and advanced computational modeling, they reconstructed the atomic-resolution structure of polyQ and HTTex1 fibrils. This interdisciplinary approach bridges experimental and theoretical methods, offering a comprehensive understanding of these unique protein clumps.
The research revealed that the polyQ fibril core consists of stacked β-sheets, each formed by antiparallel β-strands with minimal turns. These sheets are tightly packed, devoid of water, and exhibit a repetitive, pseudo-symmetric arrangement.
Unlike other amyloid fibrils, polyQ structures lack the twisting patterns commonly observed in parallel in-register β-sheets. Instead, their architecture is characterized by “glutamine zippers” and hydrogen-bonded ladders, which stabilize the fibril core.
Related Stories
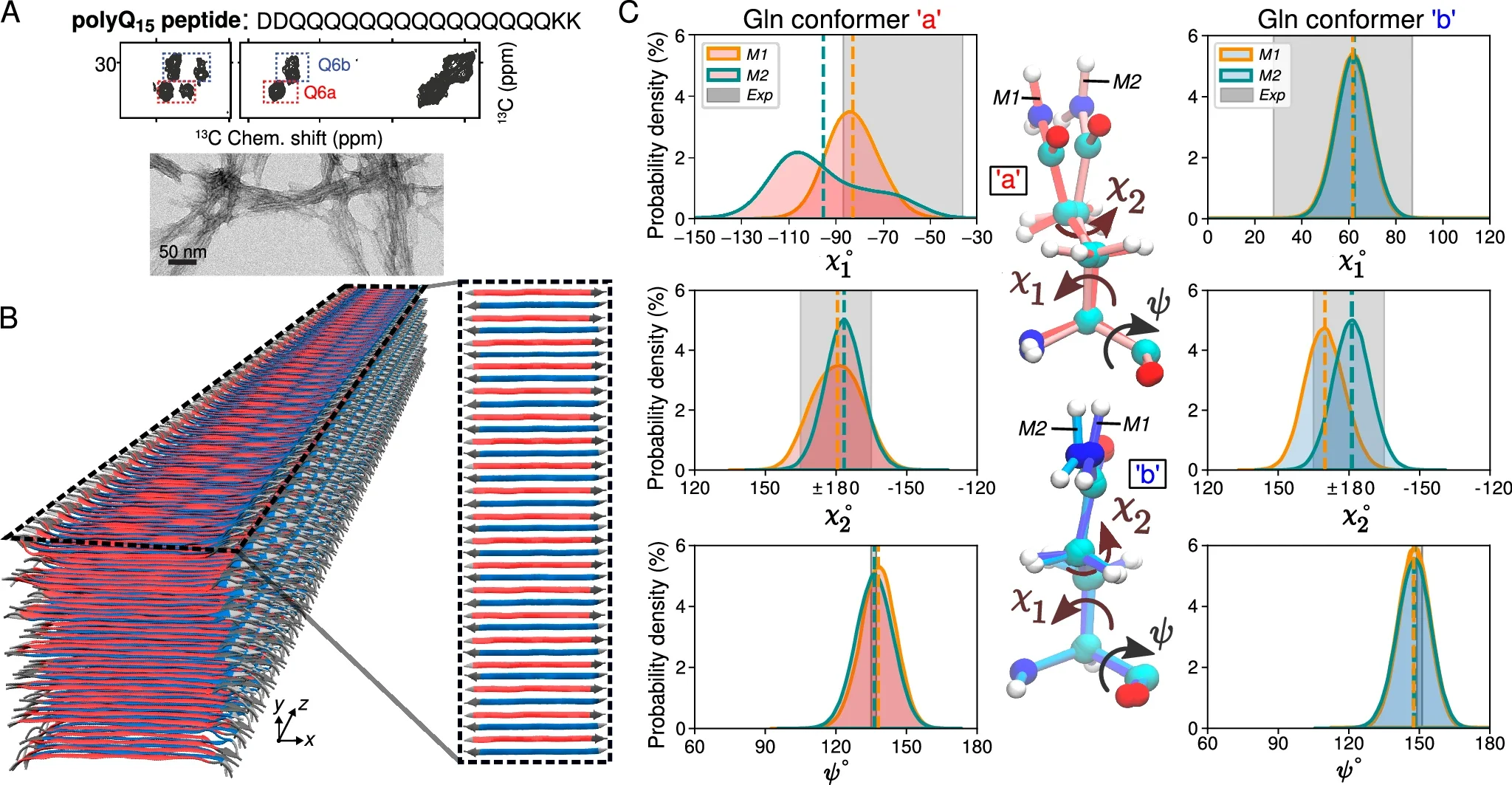
Using molecular dynamics simulations and ssNMR data, researchers identified two dominant glutamine conformations within the fibril core. These conformations, referred to as “a” and “b,” alternate in a repeating pattern, creating a distinctive spectroscopic fingerprint. This fingerprint allows for precise characterization of the fibril structure and offers potential targets for diagnostic imaging.
The molecular modeling revealed that the polyQ core is formed by an infinitely repeating eight-glutamine unit. This structural motif is notable for its translational symmetry and the absence of the twisting typically seen in amyloid fibrils.
Furthermore, the structure fulfills critical constraints required for stability, including specific dihedral angles that enable interdigitating hydrogen bonds between side chains. These findings underscore the distinctiveness of HD-related fibrils and their deviation from conventional amyloid structures.
Understanding the structure of HTTex1 fibrils has significant implications for HD research. The molecular architecture provides insights into the biological properties of these fibrils, including their resistance to degradation and their role in neurotoxicity.
For instance, the densely packed core and limited solvent accessibility hinder post-aggregation polyubiquitination, complicating the cellular mechanisms for fibril clearance.
Moreover, the detailed structural data enable the design of targeted ligands for imaging and diagnostics. These ligands could help detect fibril formation in vivo, offering earlier and more accurate diagnoses of HD. Additionally, the findings may guide the development of therapeutic inhibitors or modulators to disrupt fibril formation and progression.
Markus Miettinen emphasized the importance of this research, stating, “Understanding the structure of protein clumps is a crucial piece of the puzzle in understanding how these proteins cause disease. Our new molecular findings are essential for further developing diagnostic tools and imaging techniques to detect and monitor disease proteins in patients.”
This groundbreaking study was made possible by a collaboration of researchers from institutions across Europe. Contributors included Mahdi Bagherpoor Helabad from the Max Planck Institute of Colloids and Interfaces, Irina Matlahov and colleagues from the University of Groningen, and several others.
Their findings, published in Nature Communications, represent a milestone in HD research and underscore the value of interdisciplinary approaches in tackling complex biological problems.
Funding for this research came from foundations supporting Huntington’s disease studies, with significant contributions from families affected by the disease. Their support highlights the critical role of public and private partnerships in advancing scientific discoveries.
The study also highlights the broader applicability of integrative structural biology. While the primary focus is on HD, the methods employed could be extended to other neurodegenerative diseases characterized by amyloid fibrils.
Alzheimer’s and Parkinson’s diseases, for example, involve protein aggregates with distinct structures and properties. By leveraging the tools and techniques developed for studying polyQ fibrils, researchers may uncover new therapeutic targets across a range of conditions.
While Huntington’s disease is the primary focus, the methods and findings extend to other neurodegenerative diseases. Protein clumping is a common feature in disorders like Alzheimer’s and Parkinson’s, though the structures differ significantly. The unique properties of HD-related fibrils raise intriguing questions about their formation mechanisms and biological roles.
In addition to offering hope for HD patients, the research contributes to the broader field of molecular biology. The tools and techniques developed in this study are expected to facilitate further breakthroughs in structural biology, paving the way for innovative diagnostic and therapeutic strategies.
For instance, the use of molecular simulations to complement experimental data represents a significant advancement in the ability to model complex biological systems accurately.
Markus Miettinen highlighted the broader impact of this work, noting, “Beyond the new insights into Huntington’s disease, we have developed tools that make molecular simulations more accessible to researchers worldwide. This interdisciplinary approach is the future of structural biology.”
As research progresses, the understanding of HD-related fibrils may also inform efforts to mitigate other amyloid-related diseases. The distinct structural features of polyQ fibrils, such as their block-like core and antiparallel β-sheet arrangement, could serve as a model for studying other atypical amyloid structures.
This knowledge has the potential to revolutionize the field of neurodegenerative disease research, offering new avenues for intervention and treatment.
The findings underscore the importance of continued investment in basic and applied research. By addressing the molecular underpinnings of diseases like HD, scientists can develop targeted strategies to alleviate suffering and improve quality of life for patients worldwide.
The collaborative nature of this work serves as a testament to the power of interdisciplinary science in overcoming complex challenges.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Scientists make major new discovery in fight against Huntington’s disease appeared first on The Brighter Side of News.