In labs focused on clean energy, a quiet shift is underway. Instead of treating carbon dioxide as a dead-end waste gas, researchers want to turn it into a useful starting point. A new study from Vrije Universiteit Brussel describes progress toward solar fuel systems that can convert sunlight into chemical energy more efficiently, while lasting longer under real operating conditions.
The work centers on semiconductors, materials that can absorb light and help drive chemical reactions. In solar fuel devices, these materials sit at the heart of the system. They catch sunlight, create electrical charges, and push those charges toward electrodes where chemistry happens. If any step falters, the whole promise of solar fuels fades.
The researchers report they learned how to make these semiconductor-based systems both sturdier and stronger. They tracked how energy inside the material connects with electrodes, how charges cross key boundaries, and which factors most affect long-term stability. They also found that adding special catalysts can raise performance and extend system lifetime.
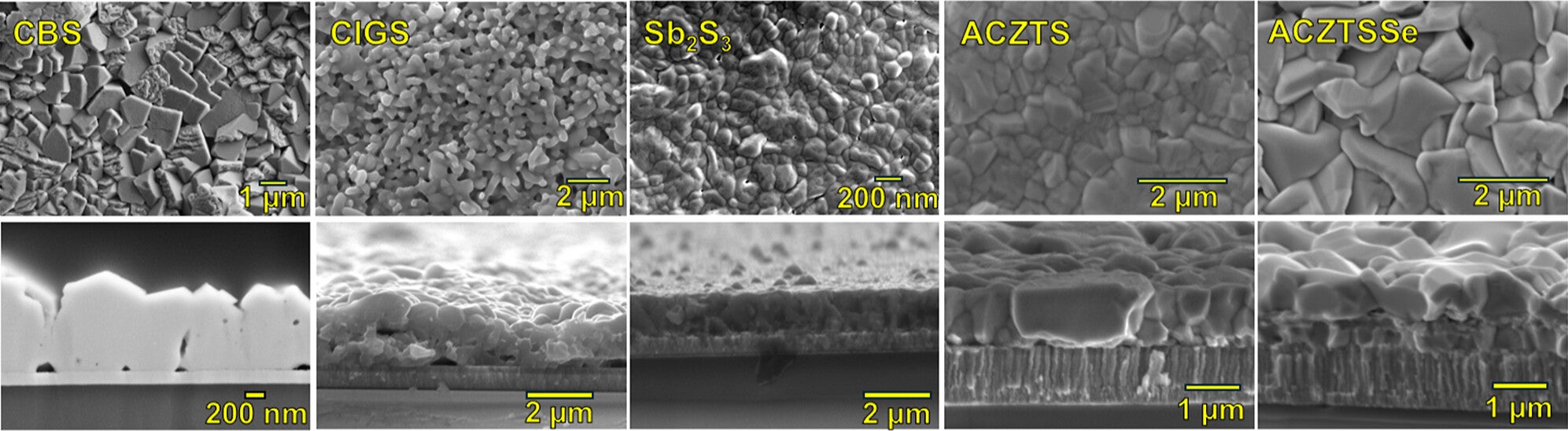
If you picture a solar fuel device as a relay team, semiconductors run the first leg. They absorb sunlight and create charges that must move quickly. Those charges then need a clean handoff to electrodes. If the handoff goes poorly, energy gets lost and the reaction slows.
The study shows how carefully mapping these interactions can reveal where losses occur. By understanding how charges travel across the system, the team could identify changes that improve efficiency. That efficiency matters because solar fuel systems must do more than work once. They need to keep working day after day.
Durability plays an equally large role. A system that performs well but fails early does not solve the real problem. The researchers describe how they strengthened stability by identifying what degrades the materials over time. They then used that insight to guide improvements that help the device keep its performance longer.
Catalysts act like helpers at the finish line. They make the key chemical steps easier. In these systems, catalysts can help speed up reactions at the electrodes. That can reduce stress on the semiconductor and improve overall output.
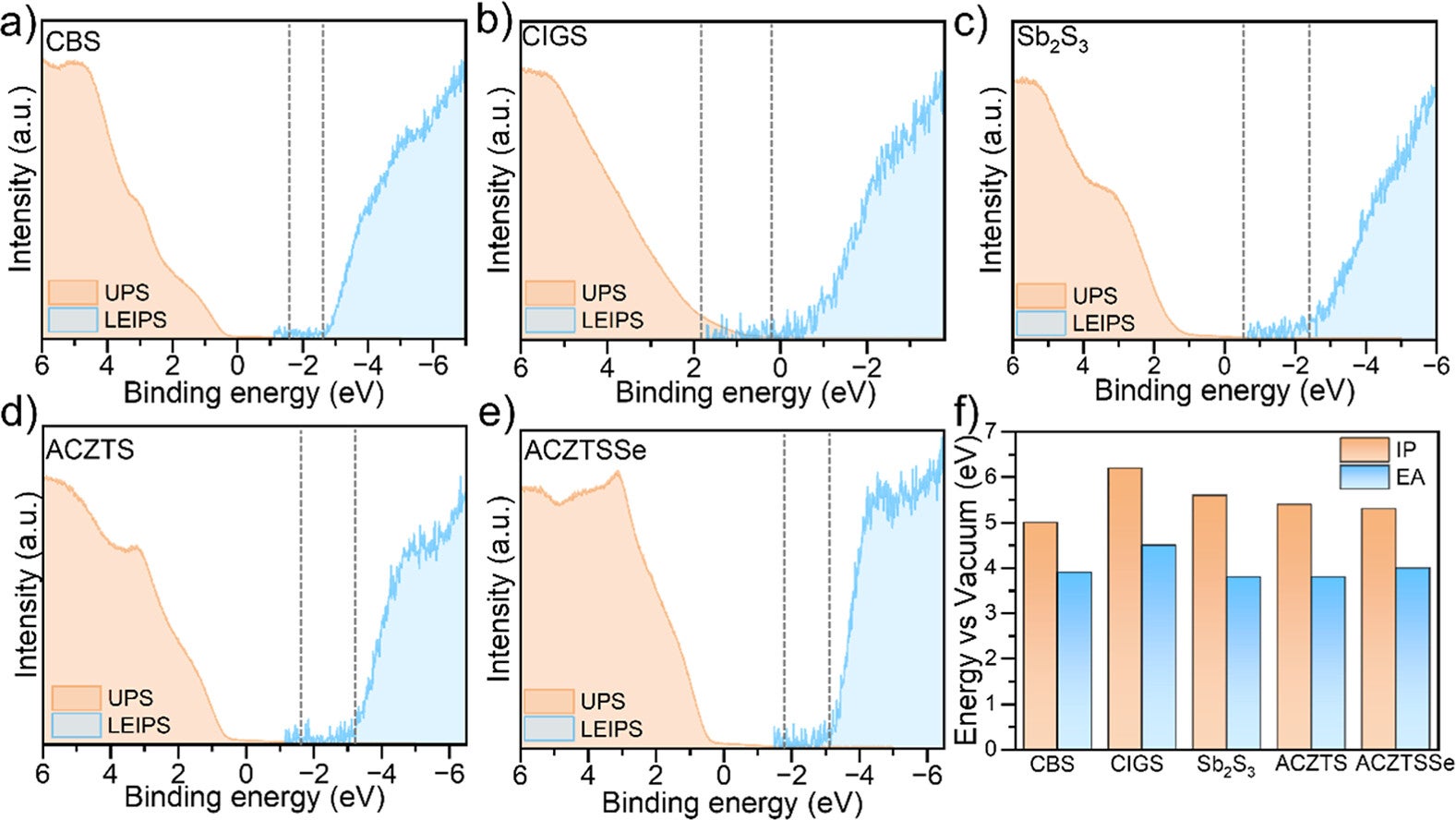
The researchers report that adding special catalysts did two things at once. It boosted performance and extended lifetime. That pairing matters because it tackles two common frustrations in solar fuel research. Many designs either work well briefly or last longer but run weak.
With better semiconductor behavior and catalytic support, the system becomes more practical. You get a clearer path for charges to travel. You also get a surface that pushes the chemistry along instead of slowing it down.
The study points toward a future where carbon dioxide can be reused as a raw material for fuel production. That idea carries emotional weight because CO₂ sits at the center of climate anxiety. It is often framed as an unavoidable byproduct. This work frames it as something you may one day feed into a device that runs on sunlight.
In the short term, the researchers emphasize the value of new knowledge. Better understanding of charge movement, electrode interactions, and stability gives engineers more reliable design rules. That can speed development of clean, affordable energy technologies.
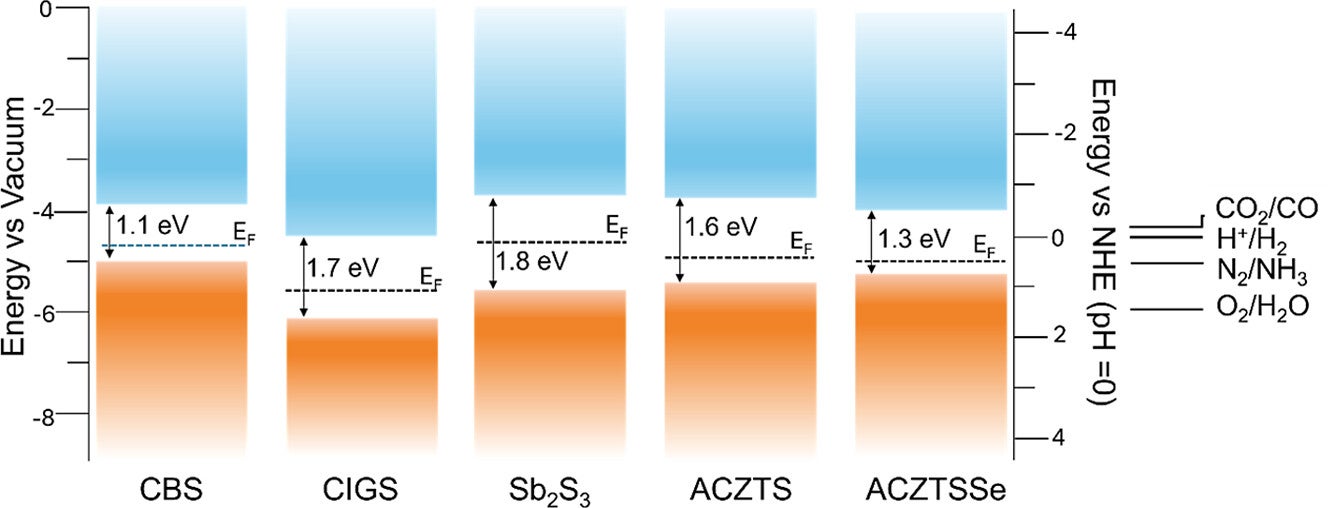
In the long term, the team sees solar fuel systems evolving into decentralized units. Instead of depending only on large power centers, you could imagine smaller systems producing solar fuels closer to where energy is needed. That vision ties to energy independence, local resilience, and progress toward climate targets.
“Our findings show that it is possible to build solar fuel systems with abundant, environmentally friendly materials that are both efficient and sustainable,” Beatriz de la Fuente from Vrije Universiteit Brussel told The Brighter Side of News. “This is a crucial step in turning CO₂ from a problem into a valuable resource,” she continued.
The research took place within a highly collaborative academic and industrial ecosystem. Funding support came from the Research Foundation Flanders (FWO). The project also forms part of SYNCAT, short for SYNergetic Design of CATalytic Materials for Integrated Photoand Electrochemical CO2 Conversion Processes.
SYNCAT is a multi-university effort supported by the Flemish Moonshot Initiative. That initiative focuses on Strategic Basic Research for Clusters and is funded by VLAIO. Together, these layers of support aim to move promising science toward real impact.
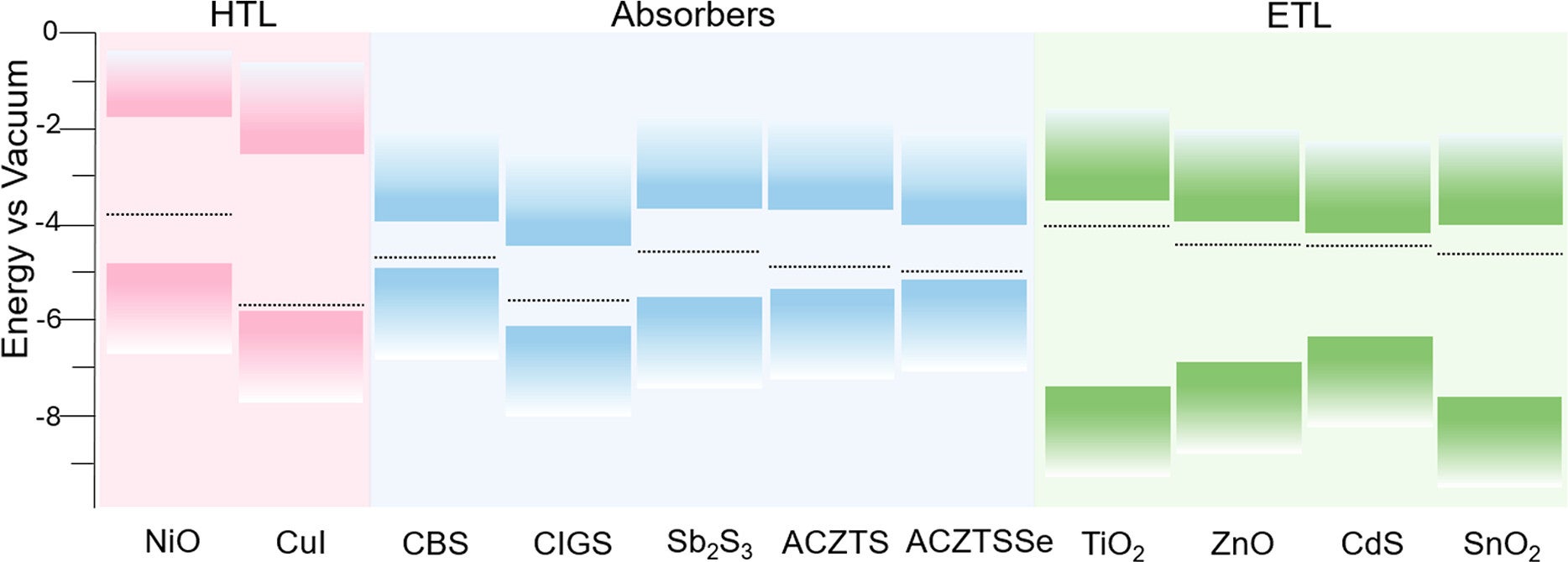
The study also highlights Vrije Universiteit Brussel’s role in sustainable and innovative energy technologies. Yet the broader message reaches beyond any one institution. If you want solar fuels to move from idea to infrastructure, you need materials that are both strong and scalable.
This research helps clarify how to build solar fuel systems that perform well and remain stable over time. By mapping how energy and charges move between semiconductors and electrodes, researchers can design devices with fewer losses and longer lifetimes.
The work also suggests that carefully chosen catalysts can improve output while reducing degradation. That combination can speed the path toward systems that run reliably outside the lab.
Over time, these advances may support technologies that reuse CO₂ as a feedstock for fuel production. If the systems become practical and widespread, they could contribute to cleaner energy options, greater energy independence, and progress toward climate goals.
Research findings are available online in The Journal of Physical Chemistry C.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Scientists use semiconductors and sunlight to convert waste carbon dioxide into fuel appeared first on The Brighter Side of News.