The intricate mechanisms behind genetic disorders are reshaping how we understand human disease. Inborn errors of immunity (IEIs), a group of monogenic disorders, exemplify this complexity. These conditions arise from genetic defects impairing innate or adaptive immune functions, leading to heightened susceptibility to infections, autoimmunity, allergies, or malignancies.
Over 450 genes have been identified as contributors to IEIs, yet their clinical manifestations can vary widely, often defying traditional genetic expectations.
Unlike many genetic disorders, IEIs often display incomplete penetrance, where individuals with disease-causing mutations remain asymptomatic. This phenomenon—estimated to affect over 20% of IEIs—complicates diagnosis and treatment. Clinical penetrance ranges from complete, where mutations always lead to disease, to incomplete, as seen in families where members carrying the same mutation exhibit disparate health outcomes.
The variability in IEIs extends to cellular behavior. Even cells sharing identical mutations can present differing immunological characteristics. Scientists have proposed several explanations, including environmental factors, modifier genes, and adaptive immune mechanisms. However, these theories don’t fully account for phenomena like discordant disease outcomes in identical twins, highlighting the need for deeper investigation.
Incomplete penetrance is not merely a theoretical curiosity. It has profound implications for understanding and managing genetic disorders. For instance, diagnostic approaches that rely solely on genetic testing may overlook individuals who carry mutations but show no symptoms.
Similarly, family members with the same mutation may require personalized treatment plans based on their unique phenotypic expressions. This underscores the importance of integrating genetic insights with clinical observations to provide comprehensive care.
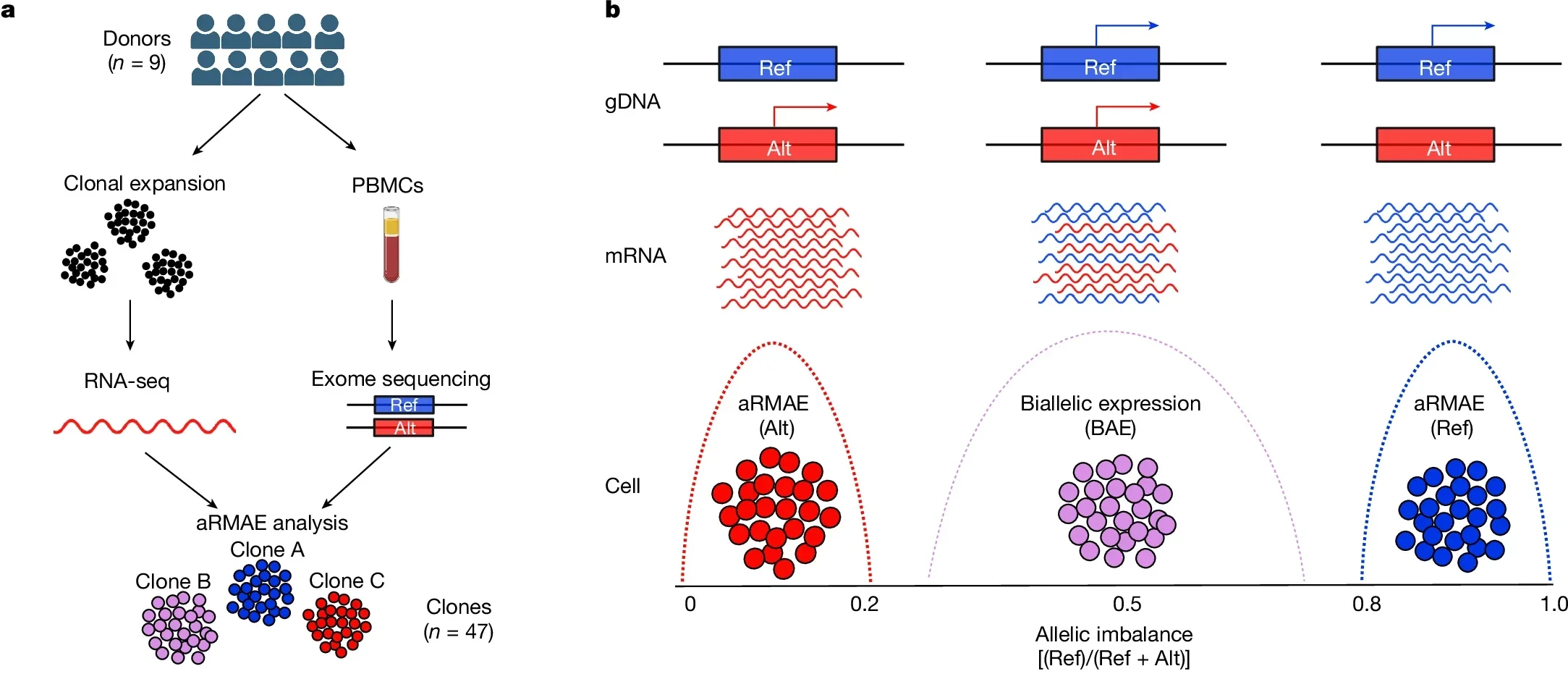
Recent research published in the journal, Nature, is shedding light on a previously underappreciated genetic mechanism: autosomal random monoallelic expression (aRMAE). This process involves the random activation of one parental allele in certain genes, resulting in a heterogeneous cell population regarding allele expression.
Unlike well-known forms of monoallelic expression, such as X chromosome inactivation, aRMAE occurs randomly, is independent of parental origin, and remains stable across cell divisions.
Related Stories
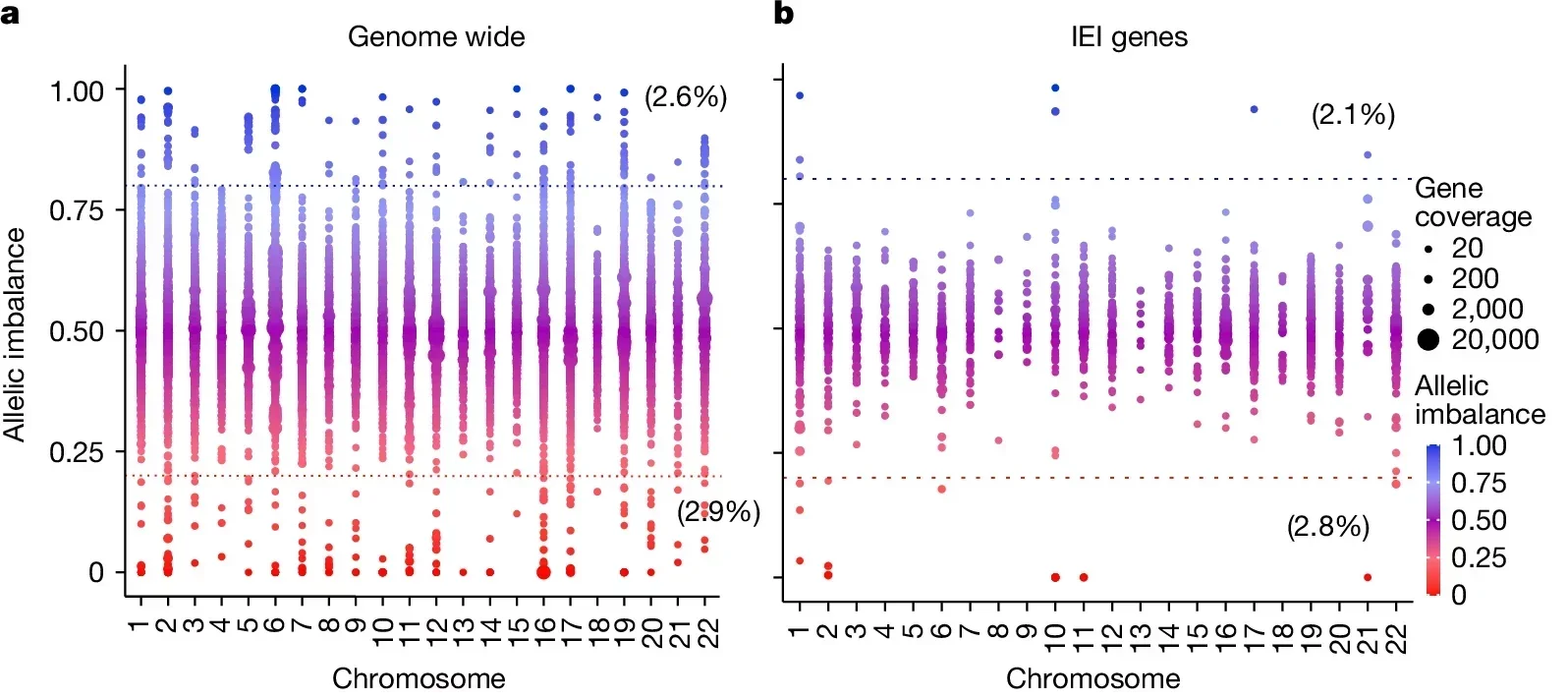
A groundbreaking study analyzed more than 4,000 genes, finding that 2% to 10% of autosomal genes undergo aRMAE. Notably, 4.3% of IEI-related genes exhibited this phenomenon. The study employed clonal primary T cells from healthy donors to trace aRMAE patterns, revealing a potential link between this mechanism and incomplete penetrance in IEIs.
This random commitment to allele expression is significant because it introduces variability at the cellular level. For example, two cells with the same genetic mutation might express different alleles, resulting in divergent functional outcomes. This variability helps explain why some individuals with a genetic predisposition to disease remain unaffected, while others develop severe symptoms.
Researchers at Columbia University used advanced techniques to explore how aRMAE might influence disease outcomes. They expanded single CD4+ or CD8+ T cells from healthy donors to create clonal populations, enabling precise tracking of allele expression. By sequencing DNA and RNA, they identified genes exhibiting significant allelic imbalance, defined as one allele contributing at least 80% of expression.
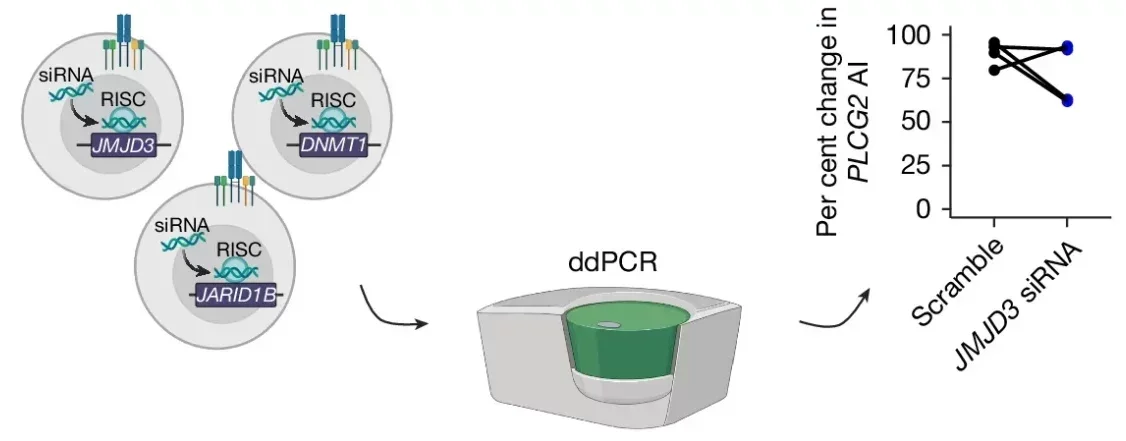
In families affected by IEIs, the researchers documented cases where aRMAE altered disease presentation. For instance, in one family, individuals with identical mutations in the PLCG2 gene showed starkly different immune phenotypes. Affected members exhibited selective expression of the mutant allele, while unaffected relatives predominantly expressed the wild-type allele. Similar patterns were observed in families with mutations in genes like STAT1 and CARD11.
These findings suggest that aRMAE might explain why some carriers of disease-causing mutations remain healthy. Study leader Dr. Dusan Bogunovic emphasized that this discovery challenges the traditional understanding of genetic inheritance: “This is suggesting that there is more plasticity in our DNA than we thought before.”
Further research into aRMAE revealed its broader implications. The phenomenon isn’t limited to immune-related genes. It was found to influence various biological systems, indicating that aRMAE might play a role in conditions as diverse as autoimmune disorders, neurodegenerative diseases, and even certain cancers. This raises exciting possibilities for uncovering new therapeutic targets across multiple medical disciplines.
The implications of aRMAE extend far beyond IEIs. The selective activation of maternal or paternal alleles could influence various genetic disorders, from lupus to cancer. Diseases with episodic flares or environmental triggers might also be linked to this phenomenon. As Dr. Bogunovic noted, “This could be just the tip of the iceberg.”
The study also highlights the importance of considering RNA—not just DNA—in understanding genetic diseases. Current diagnostic approaches rely heavily on genotyping, but incorporating “transcriptotyping,” or analyzing gene activity patterns, could provide a more comprehensive picture. “This changes the paradigm of testing beyond your DNA to your RNA,” Dr. Bogunovic explained.
Transcriptotyping could revolutionize personalized medicine. By identifying which alleles are active in specific tissues or under particular conditions, doctors could tailor treatments to an individual’s unique genetic and transcriptomic profile. This approach could also enhance prognostic accuracy, enabling early intervention for at-risk individuals before symptoms appear.
While clinical applications remain speculative, the ability to manipulate allele expression offers exciting possibilities. Early experiments in cell cultures demonstrate that it’s possible to shift gene activity, potentially silencing harmful alleles. If successful, this approach could transform genetic diseases into manageable conditions. For example, reactivating a silenced healthy allele in patients with monogenic disorders could mitigate or even eliminate disease symptoms.
Despite these breakthroughs, much remains to be understood about aRMAE’s role in human disease. For example, the mechanisms governing allele commitment are unclear. Histone modifications and DNA methylation have been implicated, but their precise contributions need further exploration.
Moreover, the prevalence of aRMAE in non-immune genes suggests its relevance across a broad spectrum of conditions. Understanding how this phenomenon interacts with environmental factors and other genetic regulators will be crucial. As research progresses, the hope is to translate these insights into novel diagnostic and therapeutic strategies.
One intriguing avenue for future research involves studying the interplay between aRMAE and environmental triggers. Certain diseases, such as multiple sclerosis and rheumatoid arthritis, are known to have both genetic and environmental components. Investigating whether aRMAE influences how individuals respond to environmental stimuli could provide valuable insights into disease mechanisms and potential interventions.
This emerging field underscores the complexity of genetics and the need for innovative approaches to unravel its mysteries. By expanding our focus from genotypes to dynamic gene activity, we move closer to a future where genetic diseases can be not only understood but effectively managed.
Researchers and clinicians alike are optimistic about the potential to harness these discoveries to improve patient outcomes and advance the frontiers of medicine.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post What if genetic traits favor one parent over the other? appeared first on The Brighter Side of News.