For centuries, the European honeybee has given people honey, wax, propolis, and even venom. Now, scientists are looking beyond the hive’s sweet rewards to explore one of its most potent and surprising gifts—a natural compound that can kill some of the deadliest breast cancer cells in record time.
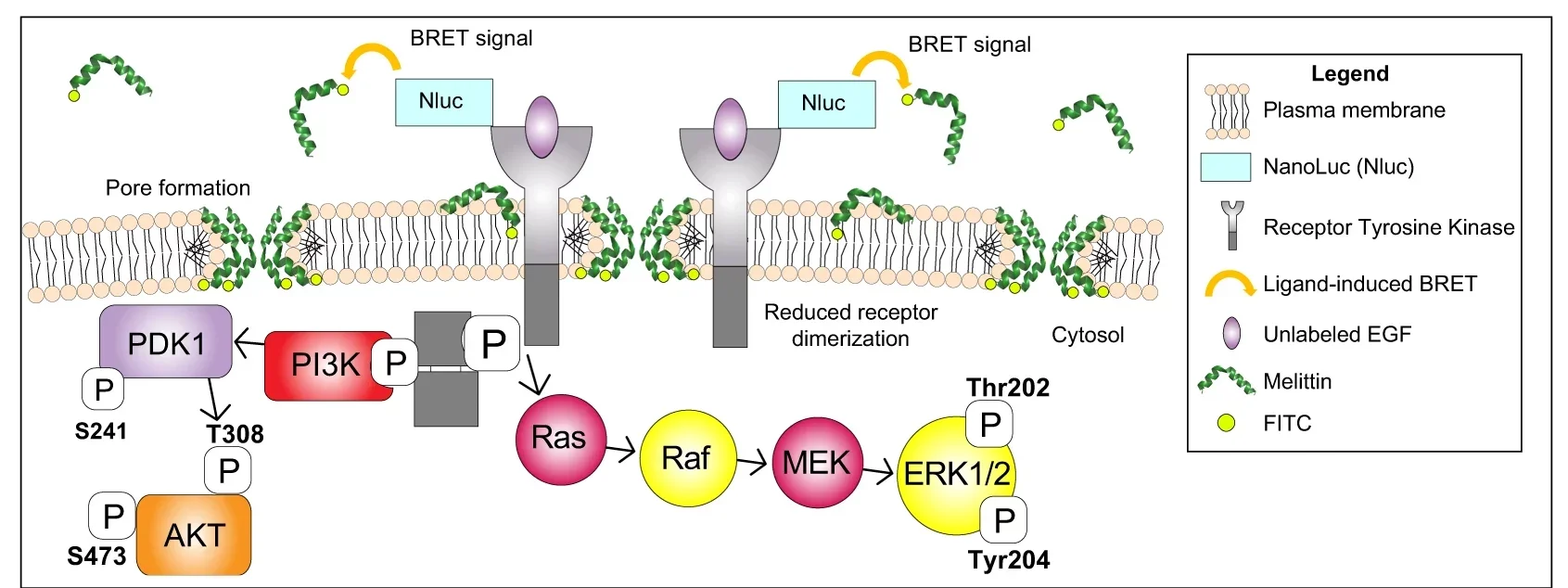
The focus is on melittin, a small peptide that makes up about half the dry weight of honeybee venom. This molecule carries a positive charge and has a unique structure that lets it attach to cell membranes. Once there, it punches tiny holes—about 4.4 nanometers wide—straight through the surface. These pores allow toxic substances to flow inside, effectively wiping out the targeted cell.
The idea of using bee venom in medicine isn’t new. First observed in 1950 for its ability to slow tumor growth in plants, its potential in cancer therapy has gained renewed interest in the past two decades. What’s changed is how deeply researchers can now study its molecular structure and its selective attack on cancer cells.

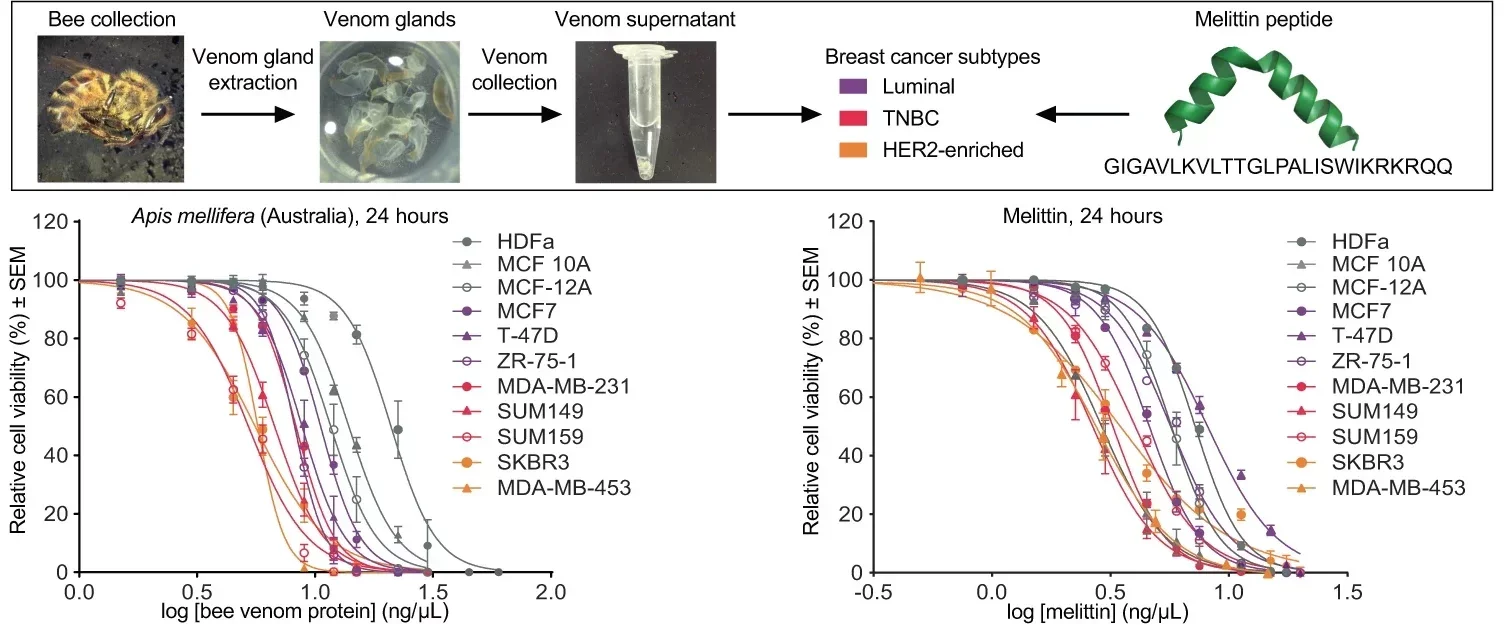
At the Harry Perkins Institute of Medical Research in Western Australia, Dr. Ciara Duffy and her colleagues collected venom from over 300 honeybees in Australia, Ireland, and England. They tested it against several breast cancer subtypes, with a focus on triple-negative breast cancer (TNBC) and HER2-enriched cancers—two aggressive forms that resist many treatments.
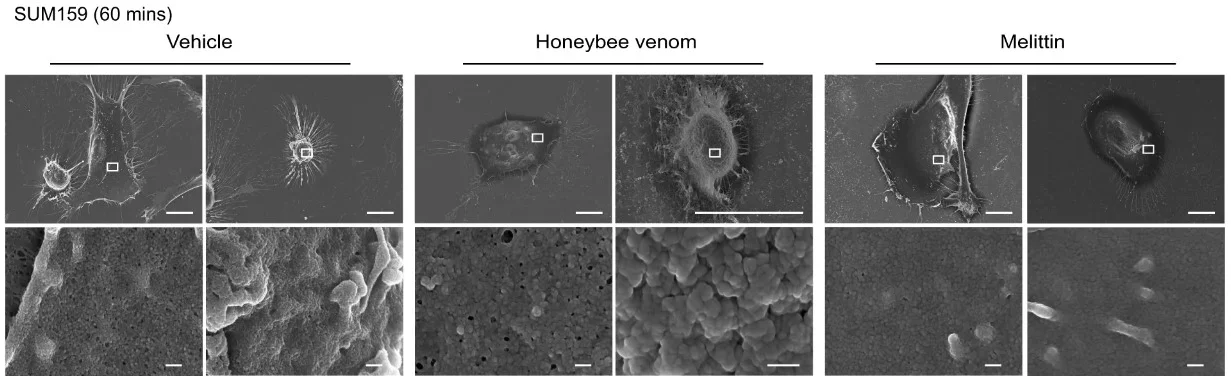
Their findings, published in NPJ Nature Precision Oncology, were striking. Melittin wiped out cancer cells within 60 minutes, while healthy cells were mostly unharmed. “The venom was extremely potent,” Dr. Duffy said. At the right concentration, it achieved 100% cancer cell death in lab tests without significant damage to normal tissue.
Even more remarkable, melittin quickly shut down chemical signals that cancer cells need to grow and divide. Within 20 minutes, it blocked the epidermal growth factor receptor (EGFR) in TNBC cells and HER2 in HER2-positive cancers—two proteins that drive uncontrolled tumor growth.
Western Australia’s Chief Scientist, Professor Peter Klinken, called the discovery “incredibly exciting,” noting that it offers “another wonderful example of where compounds in nature can be used to treat human diseases.”
Melittin’s amphipathic design—meaning it has both water-attracting and water-repelling regions—allows it to slip into a cell’s lipid membrane. Once inside, it forms toroidal pores that not only kill the cell directly but also create a gateway for other treatments.
In the study, combining melittin with the chemotherapy drug docetaxel improved drug delivery and boosted its cancer-fighting effects. Tumors in mice shrank significantly more when given the combination than with chemotherapy alone.
This opens the possibility of using melittin as a “molecular key” to enhance existing cancer therapies, especially in cases where tumors have developed drug resistance.
Triple-negative breast cancer accounts for 10–15% of all breast cancers and is among the hardest to treat. It lacks estrogen and progesterone receptors, as well as HER2, leaving few targets for current therapies. About half of TNBC cases overexpress EGFR, but drugs aimed at blocking EGFR have shown limited success because cancer cells often bypass the blocked pathway.
HER2-enriched cancers have benefited from targeted drugs like trastuzumab, but resistance eventually develops, making long-term control difficult. This makes the search for new strategies urgent—and melittin’s dual ability to directly destroy cells and block key growth signals is a promising approach.
The anticancer potential of honeybee venom goes beyond breast cancer. Past studies have shown melittin can trigger cancer cell death in melanoma, lung cancer, glioblastoma, leukemia, ovarian cancer, and pancreatic cancer. It’s also been used in nanoparticle form to limit liver metastases by influencing immune cells in the liver.
When paired with other treatments, bee venom has shown additive or even synergistic effects. For example, it has worked with cisplatin in cervical and laryngeal cancers, and with plasma-treated solutions in breast cancer and melanoma cell studies. Its effects are often linked to blocking cancer-promoting pathways such as PI3K/Akt/mTOR in breast cancer, MAPK in melanoma, JAK2/STAT3 in ovarian cancer, and NFκB in lung carcinoma.
While there are about 20,000 bee species worldwide, not all venoms are created equal. Dr. Duffy’s work found that bumblebee venom—despite containing secretory phospholipase A2—lacked melittin and did not kill breast cancer cells, even at high doses. Honeybees from Australia, Ireland, and England, however, showed nearly identical cancer-fighting power.
As with many early-stage discoveries, this research has so far been limited to laboratory and animal models. There’s still a long way to go before melittin could become part of routine cancer care. Scientists need to determine the safest delivery methods, understand potential side effects, and establish the maximum tolerated doses in humans.
Still, the promise is undeniable. Dr. Marilena Tauro, a breast cancer researcher at Moffitt Cancer Center, cautions that not every lab success translates to the clinic. Yet she remains optimistic: “Nature is a great supplier of active elements, and chemical synthesis has made it possible to provide many drugs of natural origin in the dosage required for therapeutic use.”
The ability to reproduce melittin synthetically means that large-scale production wouldn’t depend on beekeeping alone—a crucial advantage for any future therapy.
The potential of bee venom in cancer therapy shows how nature can inspire medical breakthroughs. Roughly half of all current drugs come from natural products, whether directly extracted or synthetically reproduced. Melittin’s unique properties—selective targeting, rapid action, and synergy with existing drugs—make it a strong candidate for further research.
If future trials confirm its safety and effectiveness, melittin could become part of a new class of treatments aimed at aggressive, treatment-resistant cancers. For now, its story serves as a reminder that some of the most powerful medical tools may already exist in the natural world—hidden in plain sight, perhaps even in the buzzing of a bee.
Breast cancer develops when abnormal cells in breast tissue begin growing uncontrollably, most commonly originating either in the milk ducts (ductal carcinoma) or the lobules that produce milk (lobular carcinoma). It can occur in both women and, less commonly, men, and early detection isn’t always straightforward.
Common warning signs include discovering a new lump or thickened area in the breast or underarm. These palpable masses are often firm, fixed and painless, although some may be tender or movable. Notably, many lumps turn out to be benign cysts or fibrous tissue, but any new or persistent lump deserves medical evaluation.
Beyond lumps, several more subtle changes in breast appearance or feel may signal underlying cancer:
While breast pain is rarely an early symptom, it may occur if a tumor presses on nerves or in inflammatory breast cancer. In such cases, pain may be accompanied by swelling, warmth, redness, or skin thickening.
Risk factors fall into several broad categories, most of which increase estrogen exposure or genetic susceptibility. Age is a key factor—risk rises significantly as one gets older. Other hormonal influences include early menstruation (before puberty) and late menopause, which extend lifetime exposure to estrogen.
Choices such as delayed childbirth, having fewer or no children, and shorter duration of breastfeeding also increase risk. Hormonal treatments, including certain contraceptives or prolonged hormone replacement therapy, may add a slight risk—but typically only during active use.
Lifestyle-related contributors include obesity (especially weight gain after menopause), lack of physical activity, and alcohol intake—each associated with a modest but meaningful increase in breast cancer risk. Some dietary patterns and environmental exposures—like endocrine-disrupting chemicals (e.g. BPA), secondhand smoke, and radiation—are under investigation and may add to risk.
Genetic predisposition accounts for about 5–10% of cases. Inherited mutations in genes like BRCA1, BRCA2, PALB2, PTEN, and others dramatically increase lifetime risk when present, and can influence decisions around prevention or screening.
Certain special forms of breast-related conditions also matter: ductal carcinoma in situ (DCIS) is a non-invasive “stage 0” lesion found in the lining of ducts. It often causes no symptoms and is detected through mammography via calcium deposits—but can progress to invasive cancer in some cases. Other rare forms include inflammatory breast cancer and Paget’s disease of the breast, which involve distinctive skin changes—such as redness, scaling or crusting.
Overall, early detection hinges on awareness of these warning signs and timely medical evaluation. While not all changes indicate cancer, prompt attention to lumps, skin or nipple texture alterations, persistent discharge, or unexpected breast pain improves chances of identifying disease at an early, more treatable stage.
Always consult your doctor or other qualified healthcare provider with any questions you may have regarding a medical condition, procedure, or treatment, whether it is a prescription medication, over-the-counter drug, vitamin, supplement, or herbal alternative.
Note: Materials provided above by The Brighter Side of News. Content may be edited for style and length.
Like these kind of feel good stories? Get The Brighter Side of News’ newsletter.
The post Bee venom quickly targets and kills aggressive breast cancer cells appeared first on The Brighter Side of News.